Abstract
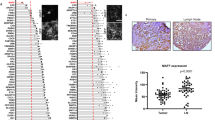
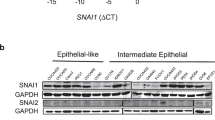
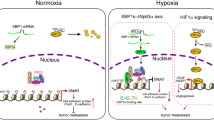
Stabilization of the hypoxia-inducible factor-1α (HIF-1α) transcription complex, caused by intratumoural hypoxia, promotes tumour progression and metastasis, leading to treatment failure and mortality in different types of human cancers. The transcription factor TWIST is a master regulator of gastrulation and mesoderm-specification and was implicated recently as an essential mediator of cancer metastasis. Notably, HIF-1α- and TWIST-null mice show similarities in their phenotypes. Here, we have shown that hypoxia or overexpression of HIF-1α promotes epithelial–mesenchymal transition (EMT) and metastastic phenotypes. We also found that HIF-1 regulates the expression of TWIST by binding directly to the hypoxia-response element (HRE) in the TWIST proximal promoter. However, siRNA-mediated repression of TWIST in HIF-1α-overexpressing or hypoxic cells reversed EMT and metastastic phenotypes. Co-expression of HIF-1α, TWIST and Snail in primary tumours of patients with head and neck cancers correlated with metastasis and the worst prognosis. These results provide evidence of a key signalling pathway involving HIF-1α and TWIST that promotes metastasis in response to intratumoural hypoxia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Gupta, G. P. & Massague, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Harris, A. L. Hypoxia — a key regulatory factor in tumour growth. Nature Rev. Cancer 2, 38–47 (2002).
Semenza, G. L. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol. Med. 8, S62–S67 (2002).
Thompson, E. W., Newgreen, D. F. & Tarin, D. Carcinoma invasion and metastasis: a role for epithelial–mesenchymal transition? Cancer Res. 65, 5991–5995 (2005).
Thiery, J. P. Epithelial-mesenchymal transitions in tumour progression. Nature Rev. Cancer 2, 442–454 (2002).
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Rev. Mol. Cell Biol. 7, 131–142 (2006).
Batlle, E. et al. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature Cell Biol. 2, 84–89 (2000).
Cano, A. et al. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biol. 2, 76–83 (2000).
Peinado, H., Olmedo, D. & Cano, A. Snail, ZEB, and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nature Rev. Cancer 7, 415–428 (2007).
Moody, S. E. et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8, 197–209 (2005).
Imai, T. et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am. J. Pathol. 161, 1437–1447 (2003).
Krishnamachary, B. et al. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 66, 2725–2731 (2006).
Evans, A. J. et al. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and Snail. Mol. Cell. Biol. 27, 157–169 (2007).
Erler, J.T., et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226 (2006).
Castanon, I. & Baylies, M. K. Twist in fate: evolutionary comparison of Twist structure and function. Gene 287, 11–22 (2002).
Furlong, E. E., Andersen, E. C., Null, B., White, K. P. & Scott, M. P. Patterns of gene expression during Drosophila mesoderm development. Science 293, 1629–1633 (2001).
Yang, J., et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Lee, T. K., et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin. Cancer Res. 12, 5369–5376 (2006).
Ryan, H. E. Lo, J., & Johnson, R. S. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 17, 3005–3115 (1998).
Iyer, N.V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12, 149–162 (1998).
Chen, Z. F. & Behringer, R. R. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 9, 686–699 (1995).
Krishnamachary, B., et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 63, 1138–43 (2003).
Huang, L. E., Gu, J., Schau, M. & Bunn, H. F. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl Acad. Sci. USA 95, 7987–7992 (1998).
Sawair, F. A., et al. Invasive front grading: reliability and usefulness in the management of oral squamous cell carcinoma. J. Oral Pathol. Med. 32, 1–9 (2003).
Hirota, K. & Semenza, G. L. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit. Rev. Oncol. Hematol. 59, 15–26 (2006).
Alexander, N.R., et al. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res. 66, 3365–3369 (2006).
Koshiji, M., et al. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 23, 1949–1956 (2004).
Janssen, H. L., Haustermans, K. M., Balm, A. J. & Begg, A. C. Hypoxia in head and neck cancer: how much, how important? Head Neck 27, 622–638 (2005).
Pugh, C. W., Gleadle, J. & Maxwell, P.H. Hypoxia and oxidative stress in breast cancer: hypoxia signalling pathways. Breast Cancer Res. 3, 313–317 (2001).
Yang, M.H., et al. Overexpression of NBS1 induces epithelial-mesenchymal transition and co-expression of NBS1 and Snail predicts metastasis of head and neck cancer. Oncogene 26, 1459–1467 (2007).
Koukourakis, M.I., et al. Endogenous markers of two separate hypoxia response pathways (hypoxia-inducible factor 2α and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J. Clin. Oncol. 24, 727–735 (2006).
Hui, E. P., et al. Coexpression of hypoxia-inducible factors 1α and 2α, carbonic anhydrase IX and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin. Cancer Res. 8, 2595–2604 (2002).
Trastour, C., et al. HIF-1α and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int. J. Cancer 120, 1451–1458 (2007).
Corsi, A. K., Kostas, S. A., Fire, A. & Krause, M. Caenorhabditis elegans Twist plays an essential role in non-striated muscle development. Development 127, 2041–2051 (2000).
Provot, S. et al. Hif-1α regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol. 177, 451–464 (2007).
Wu, K. J., et al. Direct activation of TERT transcription by c-Myc. Nature Genet. 21, 220–224 (1999).
Chiang, Y. C., Teng, S. C., Su, Y. N., Hsieh, F. J. & Wu, K. J. c-Myc directly regulates the transcription of the NBS1 gene involved in DNA double-strand break repair. J. Biol. Chem. 278, 19286–19291 (2003).
Tickoo, S. K., et al. Immunohistochemical expression of hypoxia-inducible factor-1α and its downstream molecules in sarcomatoid renal cell carcinoma. J. Urol. 177, 1258–1263 (2007).
Maestro, R., et al. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 13, 2207–2217 (1999).
Acknowledgements
We thank K. W. Chang for TMA construction, and C. H. Huang and D. H. Hsu for excellent technical assistance. We are grateful to T. Y. Chou and W. Y. Li for providing expert opinions on pathology reading and immunohistochemical analysis. This work was supported in part by National Research Program for Genomic Medicine (DOH-96-TD-G-111-002)(K.J.W.), Taipei Veterans General Hospital VGH 96-C1-126, V-96-ER2-008 (M.H.Y.), V-96-ER2-009 (S.Y.C.), National Science Council (NSC-95-2320-B-010-065)(K.J.W.); (95-2314-B-075-083)(M.H.Y.), a grant from Ministry of Education, Aim for the Top University Plan (95A-C-D01-PPG-05, 96A-D-D139)(K.J.W.), and National Health Research Institutes (NHRI-EX-96-9611BI)(K.J.W.).
Author information
Authors and Affiliations
Contributions
K. J. W., M. H. Y. and M. Z. W. conceived and designed the experiments; M. H. Y. and M. Z. W. performed the experiments with the assistance of S. H. C. for in vivo work; M. H. Y., M. Z. W., S. C. T. and K. J. W. analysed the data; K. J. W. and M. H. Y. wrote the paper with the assistance of S. C. T; S. Y. C., C. J. L., P. M. C. and M. H. Y. collected and treated samples from head and neck cancer patients.
Corresponding author
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4, S5, S6, Supplementary tables S1, S2, S3, S4, S5, S6 and Supplementary Methods (PDF 1824 kb)
Rights and permissions
About this article
Cite this article
Yang, MH., Wu, MZ., Chiou, SH. et al. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat Cell Biol 10, 295–305 (2008). https://doi.org/10.1038/ncb1691
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1691
This article is cited by
-
Redox signalling regulates breast cancer metastasis via phenotypic and metabolic reprogramming due to p63 activation by HIF1α
British Journal of Cancer (2024)
-
Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer
Experimental & Molecular Medicine (2024)
-
Single-copy Snail upregulation causes partial epithelial-mesenchymal transition in colon cancer cells
BMC Cancer (2023)
-
Systematic analysis of histone acetylation regulators across human cancers
BMC Cancer (2023)
-
Signaling pathways in cancer metabolism: mechanisms and therapeutic targets
Signal Transduction and Targeted Therapy (2023)