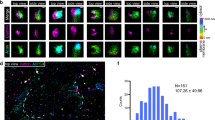
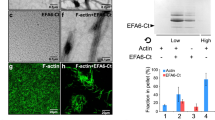
Abstract
The dynamic regulation of actin polymerization plays crucial roles in cell morphology and endocytosis. The mechanistic details of these processes and the proteins involved are not fully understood, especially in neurons. PICK1 is a PDZ–BAR-domain protein involved in regulated AMPA receptor (AMPAR) endocytosis in neurons. Here, we demonstrate that PICK1 binds filamentous (F)-actin and the actin-nucleating Arp2/3 complex, and potently inhibits Arp2/3-mediated actin polymerization. RNA interference (RNAi) knockdown of PICK1 in neurons induces a reorganization of the actin cytoskeleton resulting in aberrant cell morphology. Wild-type PICK1 rescues this phenotype, but a mutant PICK1, PICK1W413A, that does not bind or inhibit Arp2/3 has no effect. Furthermore, this mutant also blocks NMDA-induced AMPAR internalization. This study identifies PICK1 as a negative regulator of Arp2/3-mediated actin polymerization that is critical for a specific form of vesicle trafficking, and also for the development of neuronal architecture.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
da Silva, J. S. & Dotti, C. G. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nature Rev. Neurosci. 3, 694–704 (2002).
Kaksonen, M., Toret, C. P. & Drubin, D. G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nature Rev. Mol. Cell. Biol. 7, 404–414 (2006).
Stradal, T. E. & Scita, G. Protein complexes regulating Arp2/3-mediated actin assembly. Curr. Opin. Cell Biol. 18, 4–10 (2006).
Meyer, G. & Feldman, E. L. Signaling mechanisms that regulate actin-based motility processes in the nervous system. J. Neurochem. 83, 490–503 (2002).
Luo, L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 18, 601–635 (2002).
Kakimoto, T., Katoh, H. & Negishi, M. Regulation of neuronal morphology by Toca-1, an F-BAR/EFC protein that induces plasma membrane invagination. J. Biol. Chem. 281, 29042–29053 (2006).
Pinyol, R., Haeckel, A., Ritter, A., Qualmann, B. & Kessels, M. M. Regulation of N-wasp and the arp2/3 complex by abp1 controls neuronal morphology. PLoS 2, e400 (2007).
Qualmann, B., Kessels, M. M. & Kelly, R. B. Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 150, F111–F116 (2000).
Jeng, R. L. & Welch, M. D. Cytoskeleton: actin and endocytosis — no longer the weakest link. Curr. Biol. 11, R691–R694 (2001).
Merrifield, C. J. Seeing is believing: imaging actin dynamics at single sites of endocytosis. Trends Cell Biol. 14, 352–358 (2004).
Matus, A., Brinkhaus, H. & Wagner, U. Actin dynamics in dendritic spines: a form of regulated plasticity at excitatory synapses. Hippocampus 10, 555–560 (2000).
Rao, A. & Craig, A. M. Signaling between the actin cytoskeleton and the postsynaptic density of dendritic spines. Hippocampus 10, 527–541 (2000).
Dillon, C. & Goda, Y. The actin cytoskeleton: integrating form and function at the synapse. Annu. Rev. Neurosci. 28, 25–55 (2005).
Star, E. N., Kwiatkowski, D. J. & Murthy, V. N. Rapid turnover of actin in dendritic spines and its regulation by activity. Nature Neurosci. 5, 239–246 (2002).
Blanpied, T. A., Scott, D. B. & Ehlers, M. D. Dynamics and regulation of clathrin coats at specialised endocytic zones of dendrites and spines. Neuron 36, 435–449 (2002).
Lu, J. et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron 55, 874–889 (2007).
Kim, C. H., Chung, H. J., Lee, H. K. & Huganir, R. L. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc. Natl Acad. Sci. USA 98, 11725–11730 (2001).
Iwakura, Y. et al. N-methyl-D-aspartate-induced alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptor down-regulation involves interaction of the carboxyl terminus of GluR2/3 with Pick1. Ligand-binding studies using Sindbis vectors carrying AMPA receptor decoys. J. Biol. Chem. 276, 40025–40032 (2001).
Hanley, J. G. & Henley, J. M. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 24, 3266–3278 (2005).
Malinow, R. & Malenka, R. C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 (2002).
Bredt, D. S. & Nicoll, R. A. AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379 (2003).
Dawson, J. C., Legg, J. A. & Machesky, L. M. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 16, 493–498 (2006).
Itoh, T. et al. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell 9, 791–804 (2005).
Tsujita, K. et al. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J. Cell Biol. 172, 269–279 (2006).
Xia, J., Zhang, X., Staudinger, J. & Huganir, R. L. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 22, 179–187 (1999).
Rohatgi, R. et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 (1999).
Mullins, R. D., Heuser, J. A., & Pollard, T. D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad. Sci USA 95, 6181–6186 (1998).
Toshima, J., Toshima, J. Y., Martin, A. C. & Drubin, D. G. Phosphoregulation of Arp2/3-dependent actin assembly during receptor-mediated endocytosis. Nature Cell Biol. 7, 246–254 (2005).
Lu, W. & Ziff, E. B. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron 47, 407–421 (2005).
Jin, W. et al. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J. Neurosci. 26, 2380–2390 (2006).
Strasser, G. A., Rahim, N. A., VanderWaal, K. E., Gertler, F. B. & Lanier, L. M. Arp2/3 is a negative regulator of growth cone translocation. Neuron 43, 81–94 (2004).
Steinberg, J. P. et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 49, 845–860 (2006).
Ehlers, M. D. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511–525 (2000).
Zhou, Q., Xiao, M. & Nicoll, R. A. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc. Natl Acad. Sci. USA 98, 1261–1266 (2001).
Bartoe, J. L. et al. Protein interacting with C-kinase 1/protein kinase Calpha-mediated endocytosis converts netrin-1-mediated repulsion to attraction. J. Neurosci. 26, 3192–3205 (2006).
Merrifield, C. J., Feldman, M. E., Wan, L. & Almers, W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nature Cell Biol. 4, 691–698 (2002).
Merrifield, C. J., Qualmann, B., Kessels, M. M. & Almers, W. Neural Wiskott Aldrich syndrome protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur. J. Cell Biol. 83, 13–18 (2004).
Peter, B. J. et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499 (2004).
McMahon, H. T. & Gallop, J. L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590–596 (2005).
Mattila, P. K. et al. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J. Cell Biol. 176, 953–964 (2007).
Hanley, J. G., Khatri, L., Hanson, P. I. & Ziff, E. B. NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron 34, 53–67 (2002).
Acknowledgements
We thank G. Cory for invaluable discussions and advice on actin biology. We also thank A. Clarke and E. Compton-Daw for the use of and assistance with the fluorimeter, E. Ziff for the KK251,252EE construct, L. J. King, J. Mellor, J. Henley for critical reading of the manuscript. J.G.H is a fellow of the Wellcome Trust, D.R. is funded by an MRC studentship. This work was supported by ENI-NET.
Author information
Authors and Affiliations
Contributions
D.L.R. planned and performed the biochemistry and some imaging experiments. S.M. supervised generation of shRNA, planned and performed some imaging experiments. E.L.J. generated shRNA constructs. J.G.H. planned and performed imaging experiments, mutagenesis and cloning, supervised the project and wrote the paper.
Corresponding author
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4, S5, S6 and S7 (PDF 1677 kb)
Rights and permissions
About this article
Cite this article
Rocca, D., Martin, S., Jenkins, E. et al. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat Cell Biol 10, 259–271 (2008). https://doi.org/10.1038/ncb1688
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1688
This article is cited by
-
Roles of palmitoylation in structural long-term synaptic plasticity
Molecular Brain (2021)
-
Identification of Neurensin-2 as a novel modulator of emotional behavior
Molecular Psychiatry (2021)
-
Defects in syntabulin-mediated synaptic cargo transport associate with autism-like synaptic dysfunction and social behavioral traits
Molecular Psychiatry (2021)
-
Activation of the p11/SMARCA3/Neurensin-2 pathway in parvalbumin interneurons mediates the response to chronic antidepressants
Molecular Psychiatry (2021)
-
Rac1 is a downstream effector of PKCα in structural synaptic plasticity
Scientific Reports (2020)