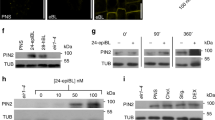
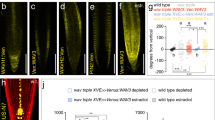
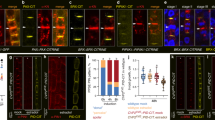
Abstract
The polarization of yeast and animal cells relies on membrane sterols for polar targeting of proteins to the plasma membrane, their polar endocytic recycling and restricted lateral diffusion1,2,3,4. However, little is known about sterol function in plant-cell polarity5. Directional root growth along the gravity vector requires polar transport of the plant hormone auxin. In Arabidopsis, asymmetric plasma membrane localization of the PIN–FORMED2 (PIN2) auxin transporter directs root gravitropism6,7,8,9,10. Although the composition of membrane sterols influences gravitropism and localization of two other PIN proteins11, it remains unknown how sterols contribute mechanistically to PIN polarity. Here, we show that correct membrane sterol composition is essential for the acquisition of PIN2 polarity. Polar PIN2 localization is defective in the sterol-biosynthesis mutant cyclopropylsterol isomerase1-1 (cpi1-1) which displays altered sterol composition, PIN2 endocytosis, and root gravitropism. At the end of cytokinesis, PIN2 localizes initially to both newly formed membranes but subsequently disappears from one. By contrast, PIN2 frequently remains at both daughter membranes in endocytosis-defective cpi1-1 cells. Hence, sterol composition affects post-cytokinetic acquisition of PIN2 polarity by endocytosis, suggesting a mechanism for sterol action on establishment of asymmetric protein localization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Keller, P. & Simons, K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 140, 1357–1367 (1998).
Bagnat, M. & Simons, K. Cell surface polarization during yeast mating. Proc. Natl Acad. Sci. USA 99, 14183–14188 (2002).
Valdez-Taubas, J. & Pelham, H. R. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr. Biol. 13, 1636–1640 (2003).
Pichler, H. & Riezman, H. Where sterols are required for endocytosis. Biochim. Biophys. Acta 1666, 51–61 (2004).
Betts, H. & Moore, I. Plant cell polarity: the ins-and-outs of sterol transport. Curr. Biol. 13, 781–783 (2003).
Luschnig, C., Gaxiola, R. A., Grisafi, P. & Fink, G. R. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12, 2175–2187 (1998).
Utsuno, K., Shikanai, T., Yamada, Y. & Hashimoto, T. AGR, an agravitropic locus of Arabidopsis thaliana, encodes a novel membrane-protein family member. Plant Cell Physiol. 39, 1111–1118 (1998).
Chen, R. et al. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl Acad. Sci. USA 95, 15112–15117 (1998).
Müller, A. et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 17, 6903–6911 (1998).
Wisniewska, J. et al. Polar PIN localization directs auxin flow in plants. Science 312, 883 (2006).
Willemsen, V. et al. Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 15, 612–625 (2003).
Petrasek, J. et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312, 914–918 (2006).
Vieten, A., Sauer, M., Brewer, P. B. & Friml, J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 12, 160–168 (2007).
Souter, M. et al. hydra mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell 14, 1017–1031 (2002).
Grebe, M. et al. Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 13, 1378–1387 (2003).
Lovato, M. A., Hart, E. A., Segura, M. J., Giner, J. L. & Matsuda, S. P. Functional cloning of an Arabidopsis thaliana cDNA encoding cycloeucalenol cycloisomerase. J. Biol. Chem. 275, 13394–13397 (2000).
Swarup, R. et al. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nature Cell Biol. 7, 1057–1065 (2005).
Sabatini, S. et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472 (1999).
Fischer, U. et al. Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr. Biol. 16, 2143–2149 (2006).
Lauber, M. H. et al. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J. Cell Biol. 139, 1485–1493 (1997).
Hartmann, M. A. & Benveniste, P. Plant membrane sterols: isolation, identification, and biosynthesis. Methods Enzymol. 148, 632–650 (1987).
Xu, J. & Scheres, B. Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17, 525–536 (2005).
Müller, I. et al. Syntaxin specificity of cytokinesis in Arabidopsis. Nature Cell Biol. 5, 531–534 (2003).
Ueda, T., Yamaguchi, M., Uchimiya, H. & Nakano, A. Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J. 20, 4730–4741 (2001).
Dhonukshe, P. et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 17, 520–527 (2007).
Schnitzer, J. E., Oh, P., Pinney, E. & Allard, J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127, 1217–1232 (1994).
Kleine-Vehn, J., Dhonukshe, P., Swarup, R., Bennett, M. & Friml, J. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18, 3171–3181 (2006).
Mongrand, S. et al. Lipid rafts in higher plant cells: purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J. Biol. Chem. 279, 36277–36286 (2004).
Borner, G. H. et al. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol. 137, 104–116 (2005).
Laloi, M. et al. Insights into the role of specific lipids in the formation and delivery of lipid microdomains to the plasma membrane of plant cells. Plant Physiol. 143, 461–472 (2007).
Acknowledgements
We gratefully acknowledge M. Bennett, D. Ehrhardt, T. Guilfoyle, J. Haseloff, R. Heidstra, R. Hellens, I. Moore, P. Mullineaux, G. Jürgens, B. Scheres, R. Swarup and J. Xu for sharing published research materials used in this study. We also acknowledge the Nottingham Arabidopsis Stock Centre for distributing mutant lines including SALK T-DNA insertion mutants, provided by J. Alonso and J. Ecker, and the John Innes Centre for EXOTIC Gene Trap lines. We thank I.-B. Carlsson and K. Lundgren for technical assistance with sterol measurements and K. Schumacher for providing seedlings for electron microscopy. We thank L. Bako, R. Bhalerao, U. Fischer, E. Johnson, A. Marchant, and G. Samuelsson for discussions and comments on the manuscript. This work was supported by a grant from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas) to M.G., in part by a postdoctoral stipend from the Carl Tryggers Foundation to Y.B., an EU Marie-Curie International Incoming Postdoctoral Fellowship to Y.I., and the Swedish Foundation for Strategic Research (SSF).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4, S5, Supplementary table S1 and Supplementary Methods (PDF 1528 kb)
Rights and permissions
About this article
Cite this article
Men, S., Boutté, Y., Ikeda, Y. et al. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol 10, 237–244 (2008). https://doi.org/10.1038/ncb1686
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1686
This article is cited by
-
Distinct mechanisms orchestrate the contra-polarity of IRK and KOIN, two LRR-receptor-kinases controlling root cell division
Nature Communications (2022)
-
WAVY GROWTH Arabidopsis E3 ubiquitin ligases affect apical PIN sorting decisions
Nature Communications (2022)
-
Phytosterol metabolism in plant positive-strand RNA virus replication
Plant Cell Reports (2022)
-
Auxin-induced signaling protein nanoclustering contributes to cell polarity formation
Nature Communications (2020)
-
Organization and dynamics of functional plant membrane microdomains
Cellular and Molecular Life Sciences (2020)