Abstract
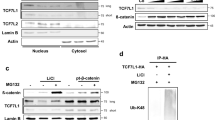
Aberrant Wnt signalling promotes oncogenesis by increasing the nuclear accumulation of β-catenin to activate downstream target genes. However, the mechanism of β-catenin recruitment to the Wnt target-gene promoter, a critical step for removing the co-repressor complex, is largely unknown. Here, we report that transducin β-like protein 1 (TBL1) and its highly related family member TBLR1 were required for Wnt–β-catenin-mediated transcription. Wnt signalling induced the interaction between β-catenin and TBL1–TBLR1, as well as their binding to Wnt target genes. Importantly, the recruitment of TBL1–TBLR1 and β-catenin to Wnt target-gene promoters was mutually dependent on each other. Furthermore, the depletion of TBL1–TBLR1 significantly inhibited Wnt–β-catenin-induced gene expression and oncogenic growth in vitro and in vivo. Our results unravel two new components required for nuclear β-catenin function, and have important implications in developing new strategies for inhibiting Wnt–β-catenin-mediated tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bienz, M. & Clevers, H. Linking colorectal cancer to Wnt signalling. Cell 103, 311–320 (2000).
Willert, K. & Jones, K. A. Wnt signalling is the party in the nucleus? Genes Dev. 20, 1394–1404 (2006).
Nelson, W. J. & Nusse, R. Convergence of Wnt, β-Catenin and cadherin pathways. Science 303, 1483–1487 (2004).
Moon, R. T., Kohn, A. D., De Ferrari, G. V. & Kaykas, A. WNT and β-catenin signalling: diseases and therapies. Nature Rev. Genet. 5, 691–701 (2004).
Morin, P. J. et al. Activation of β-catenin–Tcf signalling in colon cancer by mutations in β-catenin or APC. Science 275, 1787–1790 (1997).
Rubinfeld, B. et al. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 275, 1790–1792 (1997).
Korinek, V. et al. Constitutive transcriptional activation by a β-catenin–Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 (1997).
He, T. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998).
Roose, J. et al. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395, 608–612 (1998).
Daniels, D. L. & Weis W. I. β-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nature Cell Biol. 12, 364–371 (2005).
Billin, A. N. Thirlwell, H. & Ayer, D. E. β-catenin–histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressors to an activator. Mol. Cell. Biol. 20, 6882–6890 (2000).
Graham, T. A., Weaver, C., Mao, F., Kimelman, D. & Xu, W. Crystal structure of a β-catenin/Tcf complex. Cell 103, 885–896 (2000).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2004).
He, X., Semenov, M., Tamai, K. & Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signalling: arrows point the way. Development 131, 1663–1677 (2004).
Sierra, J., Yoshida, T., Joazeiro, C. & Jones, K. A. The APC tumor suppressor counteracts β-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 20, 586–600 (2006).
Mosimann, C., Hausmann, G. & Basler, K. Parafibromin/hyrax Activates Wnt/Wg target gene transcription by direct association with β-catenin/armadillo. Cell 125, 327–341 (2006).
Kramps, T. et al. Wnt/Wingless signalling requires BCL9/Legless-mediated recruitment of pygopus to the nuclear β-catenin–TCF complex. Cell 109, 47–60 (2002).
Matsuzawa, S. I. & Reed, J. C. Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol. Cell 7, 915–926 (2001).
Rosenfeld, M. G., Lunyak, V. V. & Glass, C. K. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 20, 1405–1428 (2006).
Barker, N. et al. The chromatin remodelling factor Brg-1 interacts with β-catenin to promote target gene activation. EMBO J. 20, 4935–4943 (2001).
Tsuda, L., Nagaraj, R., Zipursky, S. L. & Banerjee, U. An EGFR/Ebi/Sno pathway promotes delta expression by inactivating Su(H)/SMRTER repression during inductive notch signalling. Cell 110, 625–637 (2002).
Yoon, H. G. et al. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J. 22, 1336–1346 (2003).
Yoon, H. G., Choi, Y., Cole, P. A. & Wong, J. Reading and function of a histone code involved in targeting corepressor complexes for repression. Mol. Cell. Biol. 25, 324–335 (2005).
Perissi, V., Aggarwal, A., Glass, C. K., Rose, D. W. & Rosenfeld, M. G. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell 116, 511–526 (2004).
Mao, J. Low-density lipoprotein receptor-related protein-5 binds to axin and regulates the canonical Wnt signalling pathway. Mol. Cell 7, 801–809 (2001).
Zeng, W. et al. naked cuticle encodes an inducible antagonist of Wnt signalling Nature 403, 789–795 (1998).
Fang, M., Li, J., Blauwkamp, T., Bhambhani, C., Campbell, N. & Cadigan, K. M. C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. EMBO J. 25, 2735–2745 (2006).
Leung, J. Y. et al. Activation of AXIN2 expression by β-catenin-T cell factor. A feedback repressor pathway regulating Wnt signalling. J. Biol. Chem. 277, 21657–21665 (2002).
Lustig, B. et al. Negative feedback loop of Wnt signalling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 (2002).
Jho, E. H. et al. Wnt/β-catenin/Tcf signalling induces the transcription of Axin2, a negative regulator of the signalling pathway. Mol. Cell. Biol. 22, 1172–1183 (2002).
Rosin-Arbesfeld, R., Cliffe, A., Brabletz, T. & Bienz, M. Nuclear export of the APC tumour suppressor controls β-catenin function in transcription. EMBO J. 22, 1101–1113 (2003).
Townsley, F. M., Cliffem A. & Bienz, M. Pygopus and Legless target Armadillo/β-catenin to the nucleus to enable its transcriptional co-activator function. Nature Cell Biol. 6, 626–633 (2004).
Morin, P. J., Vogelstein, B. & Kinzler, K. W. Apoptosis and APC in colorectal tumorigenesis. Proc. Natl Acad. Sci. USA 93, 7950–7954 (1996).
Kolligs, F. T. et al. ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with β-catenin defects and promotes neoplastic transformation. Cancer Cell 1, 145–155 (2002).
Hovanes, K. et al. β-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nature Genet. 28, 53–57 (2001).
You, Z. et al. Wnt signalling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J. Cell Biol. 157, 429–440 (2002).
Chen, S. et al. Wnt-1 signalling inhibits apoptosis by activating β-catenin/T cell factor-mediated transcription. J. Cell Biol. 152, 87–96 (2001).
Yang, F., Zeng, Q., Yu, G., Li, S. & Wang, C. Y. Wnt/β-catenin signalling inhibits death receptor-mediated apoptosis and promotes invasive growth of HNSCC. Cell Signal. 18, 679–687 (2006).
DasGupta, R., Kaykas, A., Moon, R. T. & Perrimon, N. Functional genomic analysis of the Wnt–wingless signalling pathway. Science 308, 826–833 (2005).
Zeng, X. et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438, 873–877 (2005).
Davidson, G. et al. Casein kinase 1 γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438, 867–872 (2005).
Acknowledgements
We thank K. Cadigan for suggestions and reagents, and D. Saims and J. Guan for reading the manuscript. This work was supported by National Institutes of Health (NIH) grants to C.Y.W.
Author information
Authors and Affiliations
Contributions
J.L. performed the experiments. J.L. and C.Y.W. designed the experiments, analysed the data and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, s5 and S6 (PDF 690 kb)
Rights and permissions
About this article
Cite this article
Li, J., Wang, CY. TBL1–TBLR1 and β-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat Cell Biol 10, 160–169 (2008). https://doi.org/10.1038/ncb1684
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1684
This article is cited by
-
Desmoid Tumors: Current Perspective and Treatment
Current Treatment Options in Oncology (2024)
-
Establishment and characterization of NCC-DSM1-C1: a novel cell line derived from a patient with desmoid fibromatosis
Human Cell (2023)
-
Intraabdominal sporadic desmoid tumors and inflammation: an updated literature review and presentation and insights on pathogenesis of synchronous sporadic mesenteric desmoid tumors occurring after surgery for necrotizing pancreatitis
Clinical and Experimental Medicine (2022)
-
Overlapping Molecular Pathways Leading to Autism Spectrum Disorders, Fragile X Syndrome, and Targeted Treatments
Neurotherapeutics (2021)
-
Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities
Molecular Cancer (2020)