Abstract
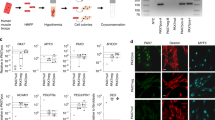
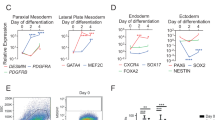
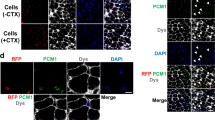
Cells derived from blood vessels of human skeletal muscle can regenerate skeletal muscle, similarly to embryonic mesoangioblasts. However, adult cells do not express endothelial markers, but instead express markers of pericytes, such as NG2 proteoglycan and alkaline phosphatase (ALP), and can be prospectively isolated from freshly dissociated ALP+ cells. Unlike canonical myogenic precursors (satellite cells), pericyte-derived cells express myogenic markers only in differentiated myotubes, which they form spontaneously with high efficiency. When transplanted into severe combined immune deficient–X-linked, mouse muscular dystrophy (scid–mdx) mice, pericyte-derived cells colonize host muscle and generate numerous fibres expressing human dystrophin. Similar cells isolated from Duchenne patients, and engineered to express human mini-dystrophin, also give rise to many dystrophin-positive fibres in vivo. These data show that myogenic precursors, distinct from satellite cells, are associated with microvascular walls in the human skeletal muscle, may represent a correlate of embryonic 'mesoangioblasts' present after birth and may be a promising candidate for future cell-therapy protocols in patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495 (1961).
Sherwood, R. I. et al. Isolation of adult mouse myogenic precursors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell 119, 543–554 (2004).
Collins, C. A. et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301 (2005).
Morgan, J. E. et al. Long-term persistence and migration of myogenic cells injected into pre-irradiated muscles of mdx mice. J. Neurol. Sci. 115, 191–200 (1993).
Beauchamp, J. R., Morgan, J. E., Pagel, C. N. & Partridge, T. A. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J. Cell Biol. 144, 1113–1121 (1999).
Montarras, D. et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064–2067 (2005).
Cossu, G. & Sampaolesi, M. New therapies for muscular dystrophy: cautious optimism? Trends Mol. Med. 10, 516–520 (2004).
Minasi, M. G. et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 129, 2773–2783 (2002).
Sampaolesi, M. et al. Cell therapy of α sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science 301, 487–492 (2003).
Armulik, A., Abramsson, A. & Betsholtz, C. Endothelial/pericyte interaction. Circ. Res. 97, 512–523 (2005).
Safadi, A., Livne, E., Silbermann, M. & Reznick, A. Z. Activity of alkaline phosphatase in rat skeletal muscle localized along the sarcolemma and endothelial cell membranes. J. Histochem. Cytochem. 39, 199–203 (1991).
Katagiri, T. et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 127, 1756–1766 (1994).
Galvez, B. G. et al. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced homing ability. J. Cell Biol. 174, 231–243 (2006).
Cossu, G. & Bianco, P. Mesoangioblasts, vascular precursors for extra-vascular mesoderm. Curr. Opin. Genet. Develop. 13, 537–542 (2003).
Neumeyer, A. M. et al. Arterial delivery of myoblasts to skeletal muscle Neurology 42, 2258–2262 (1992).
Bianco, P. & Gehron Robey, P. Marrow stromal stem cells. J. Clin. Invest. 105, 1663–1668 (2000).
Wakitani, S., Saito, T. & Caplan, A. I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 12, 1417–1426 (1995).
Asahara, T. et al. Isolation of putative precursor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Reyes, M. et al. Purification and ex vivo expansion of postnatal human marrow mesodermal precursor cells. Blood 98, 2615–2625 (2001).
Qu-Petersen, Z. et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration J. Cell Biol. 157, 851–864 (2002).
Asakura, A., Seale, P., Girgis-Gabardo, A. & Rudnicki, M. A. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 159, 123–134 (2002).
LaBarge, M. A. & Blau, H. M. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111, 589–601 (2002).
Bachrach, E. et al. Systemic delivery of human mini-dystrophin to regenerating mouse dystrophic muscle by muscle precursor cells. Proc. Natl Acad. Sci. USA 101, 3581–3586 (2004).
Torrente, Y., et al. Human circulating AC133+ stem cells replenish the satellite cell pool, restore dystrophin expression and ameliorate function upon transplantation in murine dystrophic skeletal muscle. J. Clin. Invest. 114, 182–195 (2004).
Tamaki. T. et al. Identification of myogenic-endothelial precursor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 157, 571–577 (2002).
De Bari, C. et al. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 160, 909–918 (2003).
Toma, J. G. et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nature Cell. Biol. 3, 778–784 (2001).
Rodriguez, A. M. et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J. Exp. Med. 201, 1397–1405 (2005).
Dezawa, M. et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 309, 314–317 (2005).
Luo, D., Renault, V. M. & Rando, T. A. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin. Cell. Dev. Biol. 16, 612–622 (2005).
Leong, K. G. & Karsan, A. Recent insights into the role of Notch signaling in tumorigenesis. Blood 107, 2223–2233 (2005).
Lattanzi, L. et al. High efficiency myogenic conversion of human fibroblasts by adenoviral vector-mediated MyoD gene transfer. J. Clin. Invest. 101, 2119–2128 (1998).
Wright, W. E., Shay, J. W. & Piatyszek, M. A. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 23, 3794–4005 (1995).
Ouellette, M. M. et al. Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J. Biol. Chem. 275, 10072–10076 (2000).
Li, S. et al. Stable transduction of myogenic cells with lentiviral vectors expressing a minidystrophin. Gene. Ther. 12, 1099–1108 (2005).
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Liu, W. M. et al. Analysis of high density expression microarrays with signed-rank call algorithms. Bioinformatics 18, 1593–1599 (2002).
Tusher, V. G., Tibshirani, R. & Chu,G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl Acad. Sci. USA 98, 5116–5121 (2001).
Acknowledgements
This work was supported by grants from Muscular Dystrophy Association (MDA), Telethon, Association Française contra les Myopathies (AFM), Parent Project Onlus, Cassadi Risparmio Province Lombarde (CARIPLO), Associazione Italiana ricerca sul Cancro (AIRC), EC 'Eurostemcell', 'Cellsintoorgan', MyoAmp and 'Genostem', and the Italian Ministries of Health and Research. We thank G. Arrigo for help with karyotype analysis and A. Palini for help with FACS analysis. We also thank E. Dejana for advice and for reading the manuscript.
Author information
Authors and Affiliations
Contributions
A.D. and M.S. coordinated the work and performed the in vivo transplantation and functional tests. R.T. performed the cell cultures with help from G.M. and R.M. E.T. and S.F. conducted the microarray analysis. B.S. and A.D. did the FACS work. L.P. performed the PCR and western blot analysis. A.I. and M.B. did the immunocytochemistry. B.G.G. performed the homing experiment. S.L. and J.S.C. provided the viral vectors and advice. G.P. and Y.T. provided the biological samples. W.E.W. performed the telomerase work, provide advice and revised the manuscript. P.B. and G.C. coordinated the whole project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3 and Supplementary Methods (PDF 691 kb)
Rights and permissions
About this article
Cite this article
Dellavalle, A., Sampaolesi, M., Tonlorenzi, R. et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9, 255–267 (2007). https://doi.org/10.1038/ncb1542
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1542
This article is cited by
-
Derivation and long-term maintenance of porcine skeletal muscle progenitor cells
Scientific Reports (2024)
-
Extracellular matrix: the critical contributor to skeletal muscle regeneration—a comprehensive review
Inflammation and Regeneration (2023)
-
Flow-dependent shear stress affects the biological properties of pericyte-like cells isolated from human dental pulp
Stem Cell Research & Therapy (2023)
-
SDF-1 and NOTCH signaling in myogenic cell differentiation: the role of miRNA10a, 425, and 5100
Stem Cell Research & Therapy (2023)
-
NG2-positive pericytes regulate homeostatic maintenance of slow-type skeletal muscle with rapid myonuclear turnover
Stem Cell Research & Therapy (2023)