Abstract
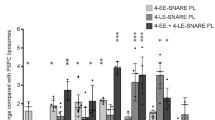
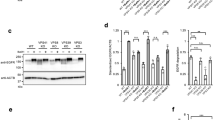
Caveolar endocytosis has an important function in the cellular uptake of some bacterial toxins, viruses and circulating proteins. However, the molecular machinery involved in regulating caveolar uptake is poorly defined. Here, we demonstrate that caveolar endocytosis is regulated by syntaxin 6, a target membrane soluble N-ethylmaleimide attachment protein receptor (t-SNARE) involved in membrane fusion events along the secretory pathway. When syntaxin 6 function was inhibited, internalization through caveolae was dramatically reduced, whereas other endocytic mechanisms were unaffected. Syntaxin 6 inhibition also reduced the presence of caveolin-1 and caveolae at the plasma membrane. In addition, syntaxin 6 inhibition decreased the delivery of GM1 ganglioside (GM1) and glycosylphosphatidylinositol (GPI)–GFP (but not vesicular stomatitis virus-glycoprotein G; VSV-G) protein from the Golgi complex to the plasma membrane. Addition of GM1 to syntaxin 6-inhibited cells resulted in the reappearance of caveolin-1 and caveolae at the plasma membrane, and restored caveolar uptake. These results suggest that syntaxin 6 regulates the delivery of microdomain-associated lipids and proteins to the cell surface, which are required for caveolar endocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Cohen, A. W., Hnasko, R., Schubert, W. & Lisanti, M. P. Role of caveolae and caveolins in health and disease. Physiol. Rev. 84, 1341–1379 (2004).
Mineo, C. & Anderson, G. W. Potocytosis. Histochem. Cell Biol. 116, 109–118 (2001).
Parton, R. G. & Richards, A. A. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4, 724–738 (2003).
Duncan, M. J., Shin, J. S. & Abraham, S. N. Microbial entry through caveolae: variations on a theme. Cell Microbiol. 4, 783–791 (2002).
Lencer, W. I., Hirst, T. R. & Holmes, R. K. Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta. 1450, 177–190 (1999).
Marjomäki, V. S., Huovila, A. -P. J., Surkka, M. A., Jokinen, I. & Salminen, A. Lysosomal trafficking in rat cardiac myocytes. J. Histochem. Cytochem. 38, 1155–1164 (1990).
Norkin, L. C. Caveolae in the uptake and targeting of infectious agents and secreted toxins. Adv. Drug Deliv. Rev. 49, 301–315 (2001).
Pelkmans, L., Kartenbeck, J. & Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nature Cell Biol. 3, 473–483 (2001).
Richterova, Z. et al. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 75, 10880–10891 (2001).
Shin, J. -S., Gao, Z. & Abraham, N. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289, 785–788 (2000).
Kim, Y. N. & Bertics, P. J. The endocytosis-linked protein dynamin associates with caveolin-1 and is tyrosine phosphorylated in response to the activation of a noninternalizing epidermal growth factor receptor mutant. Endocrinology 143, 1726–1731 (2002).
Mineo, C., Gill, G. N. & Anderson, R. G. Regulated migration of epidermal growth factor receptor from caveolae. J. Biol. Chem. 274, 30636–30643 (1999).
Shajahan, A. N. et al. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 279, 20392–20400 (2004).
Sharma, D. K. et al. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol. Biol. Cell 15, 3114–3122 (2004).
Volonte, D. et al. Flotillins–cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J. Biol. Chem. 274, 12702–12709 (1999).
Chen, Y. A. & Scheller, R. H. SNARE-mediated membrane fusion. Nature Rev. Mol. Cell Biol. 2, 98–106 (2001).
McNew, J. A. et al. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153–159 (2000).
Ungar, D. & Hughson, F. M. SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 19, 493–517 (2003).
McIntosh, D. P. & Schnitzer, J. E. Caveolae require intact VAMP for targeted transport in vascular endothelium. Am. J. Physiol. 277, H2222–H2232 (1999).
Predescu, D., Horvat, R., Predescu, S. & Palade, G. E. Transcytosis in the continuous endothelium of the myocardial microvasculature is inhibited by N-ethylmaleimide. Proc. Natl Acad. Sci. USA 91, 3014–3018 (1994).
Schnitzer, J. E., Liu, J. & Oh, P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J. Biol. Chem. 270, 14399–14404 (1995).
Kuliawat, R. et al. Syntaxin-6 SNARE involvement in secretory and endocytic pathways of cultured pancreatic β-cells. Mol. Biol. Cell 15, 1690–1701 (2004).
Perera, H. K. I. et al. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 Adipocytes. Mol. Biol Cell 14, 2946–2958 (2003).
Wendler, F., Page, L., Urbe, S. & Tooze, S. A. Homotypic fusion of immature secretory granules during maturation requires syntaxin 6. Mol. Biol. Cell 12, 1699–1709 (2001).
Ros-Baro, A. et al. Lipid rafts are required for GLUT4 internalization in adipose cells. Proc. Natl Acad. Sci. USA 98, 12050–12055 (2001).
Shigematsu, S., Watson, R. T., Khan, A. H. & Pessin, J. E. The adipocyte plasma membrane caveolin functional–structural organization is necessary for the efficient endocytosis of GLUT4. J. Biol. Chem. 278, 10683–10690 (2003).
Shewan, A. M. et al. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. Mol. Biol. Cell 14, 973–986 (2003).
Puri, V. et al. Clathrin-dependent and independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154, 535–547 (2001).
Sharma, D. K. et al. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278, 7564–7572 (2003).
Singh, R. D. et al. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 14, 3254–3265 (2003).
Olson, A. L., Knight, J. B. & Pessin, J. E. Syntaxin 4, VAMP2, and/or VAMP3–cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell Biol. 17, 2425–2435 (1997).
Tellam, J. T. et al. Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J. Biol. Chem. 272, 6179–6186 (1997).
Shubert, W. et al. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 276, 48619–48622 (2001).
Prekeris, R., Klumperman, J., Chen, Y. A. & Scheller, R. H. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J. Cell Biol. 143, 957–971 (1998).
del Pozo, M. A. et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nature Cell Biol. 7, 901–908 (2005).
Orlichenko, L., Huang, B., Krueger, E. & McNiven, M. A. EGF-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J. Biol. Chem. 281, 4570–4579 (2006).
Chamberlain, L. H., Burgoyne, R. D. & Gould, G. W. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc. Natl Acad. Sci. USA 98, 5619–5624 (2001).
Puri, V. et al. Sphingolipid storage induces accumulation of intracellular cholesterol by stimulating SREBP-1 cleavage. J. Biol. Chem. 278, 20961–20970 (2003).
Martin, L. B., Shewan, A., Millar, C. A., Gould, G. W. & James, D. E. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3-L1 adipocytes. J. Biol. Chem. 273, 1444–1452 (1998).
Volchuk, A. et al. Syntaxin 4 in 3T3-L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol. Biol. Cell 7, 1075–1082 (1996).
Morcos, P. A. Achieving efficient delivery of morpholino oligos in cultured cells. Genesis 30, 94–102 (2001).
Wendler, F. & Tooze, S. Syntaxin 6: The promiscuous behaviour of a SNARE protein. Traffic 2, 606–611 (2001).
Martin-Martin, B., Nabokina, S. M., Blasi, J., Lazo, P. A. & Mollinedo, F. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood 96, 2574–2583 (2000).
Sharma, D. K. et al. The glycosphingolipid, lactosylceramide, regulates β1-integrin clustering and endocytosis. Cancer Res. 65, 8233–8241 (2005).
Choudhury, A. et al. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 109, 1541–1550 (2002).
Martin, O. C. & Pagano, R. E. Internalization and sorting of a fluorescent analog of glucosylceramide to the Golgi apparatus of human skin fibroblasts: utilization of endocytic and nonendocytic transport mechanisms. J. Cell Biol. 125, 769–781 (1994).
Sabharanjak, S., Sharma, P., Parton, R. G. & Mayor, S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Develop. Cell 2, 411–423 (2002).
Henley, J. R., Krueger, E. W., Oswald, B. J. & McNiven, M. A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141, 85–99 (1998).
Acknowledgements
Supported by U.S. Public Health Service grant (GM-22942) to R.E.P. and by The Wellcome Trust (studentship to K.P. and Research Leave Award to G.W.G.). A.C. was supported by a fellowship from the American Heart Association. We thank L.Chamberlain (University of Glasgow, Glasgow, UK) and E. Krueger (Mayo Foundation, Rochester, MN) for helpful advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3 and S4 (PDF 707 kb)
Rights and permissions
About this article
Cite this article
Choudhury, A., Marks, D., Proctor, K. et al. Regulation of caveolar endocytosis by syntaxin 6-dependent delivery of membrane components to the cell surface. Nat Cell Biol 8, 317–328 (2006). https://doi.org/10.1038/ncb1380
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1380
This article is cited by
-
Caveolin-1 function at the plasma membrane and in intracellular compartments in cancer
Cancer and Metastasis Reviews (2020)
-
ROR1-CAVIN3 interaction required for caveolae-dependent endocytosis and pro-survival signaling in lung adenocarcinoma
Oncogene (2019)
-
Syntaxin 6: A novel predictive and prognostic biomarker in papillary renal cell carcinoma
Scientific Reports (2019)
-
Synergy in Disruption of Mitochondrial Dynamics by Aβ (1-42) and Glia Maturation Factor (GMF) in SH-SY5Y Cells Is Mediated Through Alterations in Fission and Fusion Proteins
Molecular Neurobiology (2019)
-
Glia Maturation Factor Dependent Inhibition of Mitochondrial PGC-1α Triggers Oxidative Stress-Mediated Apoptosis in N27 Rat Dopaminergic Neuronal Cells
Molecular Neurobiology (2018)