Abstract
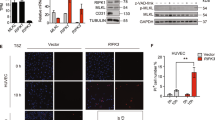
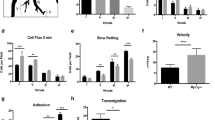
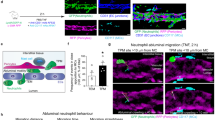
Although the adhesive interactions of leukocytes with endothelial cells are well understood, little is known about the detailed mechanisms underlying the actual migration of leukocytes across the endothelium (diapedesis). Leukocytes have been shown to use both paracellular and transcellular routes for transendothelial migration. Here we show that peripheral blood mononuclear cells (PBMCs; T- and B-lymphocytes) preferentially use the transcellular route. The intermediate filaments of both endothelial cells and lymphocytes formed a highly dynamic anchoring structure at the site of contact between these two cell types. The initiation of this process was markedly reduced in vimentin-deficient (vim−/−) PBMCs and endothelial cells. When compared with wild-type PBMCs, vim−/− PBMCs showed a markedly reduced capacity to home to mesenteric lymph nodes and spleen. Furthermore, endothelial integrity was compromised in vim−/− mice, demonstrating that intermediate filaments also regulate the barrier that governs leukocyte extravasation. Absence of vimentin resulted in highly aberrant expression and distribution of surface molecules critical for homing (ICAM-1 and VCAM-1 on endothelial cells and integrin-β1 on PBMCs). These data show that intermediate filaments are active in lymphocyte adhesion and transmigration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994).
Hogg, N. & Berlin, C. Structure and function of adhesion receptors in leukocyte trafficking. Immunol. Today 16, 327–330 (1995).
Carman, C. V. & Springer, T. A. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 167, 377–388 (2004).
Engelhardt, B. & Wolburg, H. Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur. J. Immunol. 34, 2955–2963 (2004).
Wan-Hong, S., Hsiun-ing, C. & Chauying J. J. Differential movements of VE–cadherin and PECAM-1 during transmigration of polymorphonuclear leukocytes through human umbilical vein endothelium. Blood 100, 3597–3603 (2002).
Mamdouh, Z., Chen, X., Pierini, L. M., Maxfield, F. R. & Muller, W. A. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature 421, 748–753 (2003).
Shaw, S. K., Bamba, P. S., Perkins, B. N. & Luscinskas, F. W. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 167, 2323–2330 (2001).
Colucci-Guyon, E. et al. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 79, 679–694 (1994).
Coulombe, P. A. & Wong, P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nature Cell Biol. 8, 699–706 (2004).
Chang, L. & Goldman, R. D. Intermediate filaments mediate cytoskeletal crosstalk. Nature Rev. Mol. Cell Biol. 5, 601–613 (2004).
Runembert, I. et al. Vimentin affects localization and activity of sodium–glucose cotransporter SGLT1 in membrane rafts. J. Cell Sci. 115, 713–724 (2002).
Runembert, I. et al. Recovery of Na–glucose cotransport activity after renal ischemia is impaired in mice lacking vimentin. Am. J. Physiol. Renal. Physiol. 287, F960–968 (2004).
Ameen, N. A., Figueroa, Y. & Salas, P. J. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J. Cell Sci. 114, 563–575 (2001).
Toivola, D.M. et al. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J. Cell Biol. 164, 911–921 (2004).
Ivaska, J. et al. PKCε-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 24, 3834–3845 (2005).
Brown, M. J., Hallam, J. A., Colucci-Guyon, E. & Shaw, S. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J. Immunol. 166, 6640–6646 (2001).
Marchesi, V. T. & Gowans, J. L. The migration of lymphocytes through the endothelium of venules in lymph nodes: an electron microscope study. Proc. R. Soc. London B Biol. Sci. 159, 283–290 (1964).
Ager, A. Inflammation: Border crossings. Nature 421, 703–705 (2003).
Feng, D. et al. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J. Exp. Med. 187, 903–915 (1998).
Yang, L. et al. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α activated vascular endothelium under flow. Blood 106, 584–592 (2005).
Jaffe, E. A., Nachman, R. L., Becker, C. G. & Minick, C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 52, 2745–2756 (1973).
Cooke, B. M., Morris-Jones, S., Greenwood, B. M. & Nash, G. B. Adhesion of parasitized red blood cells to cultured endothelial cells: a flow-based study of isolates from Gambian children with falciparum malaria. Parasitology 107, 359–368 (1993).
Ley, K. et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 181, 669–675 (1995).
Stolen, C. M. et al. Absence of the endothelial oxidase AOC3 leads to abnormal leukocyte traffic in vivo. Immunity 22, 105–115 (2005).
Jung, U. et al. Absence of trauma-induced leukocyte rolling in mice deficient in both P-selectin and intercellular adhesion molecule 1. J. Exp. Med. 183, 57–65 (1996).
von Andrian, U. H. et al. L-selectin function is required for α2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am. J. Physiol. 263, H1034–H1044 (1992).
Acknowledgements
We thank H. Saarrento for technical help and A. Sovikoski–Georgieva for secretarial assistance. Funding from the Academy of Finland, the Finnish Cancer Organizations, the Sigrid Jusélius Foundation, and the Association of the Finnish Life Insurance Companies is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1 and S2 (PDF 422 kb)
Supplementary Information
Supplementary Movie 1 (AVI 326 kb)
Supplementary Information
Supplementary Movie 2 (AVI 3018 kb)
Supplementary Information
Supplementary Movie 3 (AVI 3066 kb)
Supplementary Information
Supplementary Movie 4 (AVI 2825 kb)
Rights and permissions
About this article
Cite this article
Nieminen, M., Henttinen, T., Merinen, M. et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol 8, 156–162 (2006). https://doi.org/10.1038/ncb1355
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1355
This article is cited by
-
Lymphatic trafficking of immune cells and insights for cancer metastasis
Clinical & Experimental Metastasis (2023)
-
Caspase-9-mediated cleavage of vimentin attenuates the aggressiveness of leukemic NB4 cells
Molecular and Cellular Biochemistry (2023)
-
Lack of adducin impairs the stability of endothelial adherens and tight junctions and may be required for cAMP-Rac1-mediated endothelial barrier stabilization
Scientific Reports (2022)
-
Circulating Vimentin Over-Expression in Patients with Oral Sub Mucosal Fibrosis and Oral Squamous Cell Carcinoma
Indian Journal of Otolaryngology and Head & Neck Surgery (2022)
-
Enterovirus A71 capsid protein VP1 increases blood–brain barrier permeability and virus receptor vimentin on the brain endothelial cells
Journal of NeuroVirology (2020)