Abstract
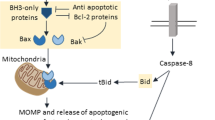
Activation of 'initiator' (or 'apical') caspases-2, -8 or -9 (refs 1–3) is crucial for induction of apoptosis. These caspases function to activate executioner caspapses that, in turn, orchestrate apoptotic cell death. Here, we show that a cell-permeable, biotinylated pan-caspase inhibitor (bVAD–fmk) both inhibited and 'trapped' the apical caspase activated when apoptosis was triggered. As expected, only caspase-8 was trapped in response to ligation of death receptors, whereas only caspase-9 was trapped in response to a variety of other apoptosis-inducing agents. Caspase-2 was exclusively activated in heat shock-induced apoptosis. This activation of caspase-2 was also observed in cells protected from heat-shock-induced apoptosis by Bcl-2 or Bcl-xL. Reduced sensitivity to heat-shock-induced death was observed in caspase-2−/− cells. Furthermore, cells lacking the adapter molecule RAIDD failed to activate caspase-2 after heat shock treatment and showed resistance to apoptosis in this setting. This approach unambiguously identifies the apical caspase activated in response to apoptotic stimuli, and establishes caspase-2 as a proximal mediator of heat shock-induced apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Renatus, M. et al. Dimer formation drives the activation of the cell death protease caspase 9. Proc. Natl Acad. Sci. USA 98, 14250–14255 (2001).
Boatright, K. M. et al. A unified model for apical caspase activation. Molecular Cell 11, 1–20 (2003).
Baliga, B. C, Read, S. H, Kumar, S. The biochemical mechanism of caspase-2 activation. Cell Death Differ. 11, 1234–1241 (2004).
Stennicke, H. R. et al. Caspase-9 can be activated without proteolytic processing. J. Biol. Chem. 274, 8359–8362 (1999).
Boatright, K. M. & Salvesen, G. S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15, 725–731 (2003).
Chang, D. W. et al. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 22, 4132–4142 (2003).
Scaffidi, C. et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17, 1675–1687 (1998).
Boatright, K. M. et al. Activation of caspases-8 and -10 by FLIP(L). Biochem. J. 382, 651–657 (2004).
Zheng, T. S., Hunot, S., Kuida, K. & Flavell, R. A. Caspase knockouts: matters of life and death. Cell Death Differ. 6, 1043–1053 (1999).
Duan, H. & Dixit, V. M. RAIDD is a new 'death' adaptor molecule. Nature 385, 86–89 (1997).
Bergeron, L. et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 12, 1304–1314 (1998).
Lassus, P., Opitz-Araya, X. & Lazebnik, Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297, 1352–1354 (2002).
Lin, C. F. et al. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramide and etoposide-induced apoptosis. J. Biol. Chem. 279, 40755–40761 (2004).
Tinel, A. & Tschopp, J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304, 843–846 (2004).
Troy, C. M., Stefanis, L., Greene, L. A. & Shelanski, M. L. Nedd2 is required for apoptosis after trophic factor withdrawal, but not superoxide dismutase (SOD1) downregulation, in sympathetic neurons and PC12 cells. J. Neurosci. 17, 1911–1918 (1997).
Troy, C. M. & Shelanski, M. L. Caspase-2 redux. Cell Death Differ. 10, 101–107 (2003).
Ekert, P. G., Silke, J. & Vaux, D. L. Caspase inhibitors. Cell Death Differ. 6, 1081–1086 (1999).
Kasibhatla, S. et al. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol. Cell 1, 543–551 (1998).
Petak, I. et al. Fas-dependent and -independent mechanisms of cell death following DNA damage in human colon carcinoma cells. Cancer Res. 60, 2643–2650 (2000).
Brunner, T. et al. Expression of Fas ligand in activated T cells is regulated by c-Myc. J. Biol. Chem. 275, 9767–9772 (2000).
Robertson, J. D. et al. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J. Biol. Chem. 277, 29803–29809 (2002).
Lin, C. F. et al. Bcl-2 rescues ceramide- and etoposide-induced mitochondrial apoptosis through blockage of caspase-2 activation. J. Biol. Chem. 280, 23758–23765 (2005).
Lassus, P., Opitz-Araya, X. & Lazebnik, Y. Corrections and clarifications. Science 306, 1683 (2004).
Cuende, E. et al. Programmed cell death by bcl-2-dependent and independent mechanisms in B lymphoma cells. EMBO J. 12, 1555–1560 (1993).
Kluck, R. M., Bossy-Wetzel, E., Green, D. R. & Newmeyer, D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275, 1132–1136 (1997).
Yang, J. et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275, 1129–1132 (1997).
Mosser, D. D. et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell Biol. 20, 7146–7159 (2000).
Cippitelli, M. et al. Hyperthermia enhances CD95-ligand gene expression in T lymphocytes. J. Immunol. 174, 223–232 (2005).
Berube, C. et al. Apoptosis caused by p53-induced protein with death domain (PIDD) depends on the death adapter protein RAIDD. Proc. Natl Acad. Sci. USA 102, 14314–14320 (2005).
Sohn, D., Schulze-Osthoff, K. & Janicke, R. U. Caspase-8 can be activated by interchain proteolysis without receptor-triggered dimerization during drug-induced apoptosis. J. Biol. Chem. 280, 5267–5273 (2005).
Shin, S. et al. Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J. 24, 3532–3542 (2005).
Baliga, B. C. et al. Role of prodomain in importin-mediated nuclear localization and activation of caspase-2. J. Biol. Chem. 278, 4899–4905 (2003).
Acknowledgements
We would like to thank Melissa O'Leary for her excellent technical assistance and Drs Guy Salvesen, Michael Pinkoski and Christine Bonzon for helpful discussion. We are grateful to Drs Guy Salvesen, Carol Troy, Seamus Martin, Marcus Peter, Andreas Strasser and Vanessa Marsden for their generous contribution of reagents. These studies were supported by the National Institutes of Health grants (AI40646, AI44828, AI47891, AI58422).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2 and S3 plus Supplementary Methods (PDF 168 kb)
Rights and permissions
About this article
Cite this article
Tu, S., McStay, G., Boucher, LM. et al. In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat Cell Biol 8, 72–77 (2006). https://doi.org/10.1038/ncb1340
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1340
This article is cited by
-
A Review on Caspases: Key Regulators of Biological Activities and Apoptosis
Molecular Neurobiology (2023)
-
The p53-caspase-2 axis in the cell cycle and DNA damage response
Experimental & Molecular Medicine (2021)
-
Effects of intravitreal injection of siRNA against caspase-2 on retinal and optic nerve degeneration in air blast induced ocular trauma
Scientific Reports (2021)
-
Uncovering the PIDDosome and caspase-2 as regulators of organogenesis and cellular differentiation
Cell Death & Differentiation (2020)
-
Cell penetrating caspase substrates promote survival of the transplanted cells
BMC Research Notes (2019)