Abstract
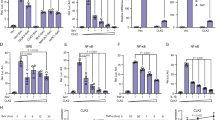
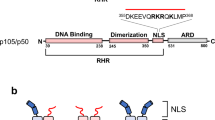
The inhibitor of NF-κB (IκB) family of proteins is believed to regulate NF-κB activity by cytoplasmic sequestration. We show that in cells depleted of IκBα, IκBβ and IκBε proteins, a small fraction of p65 binds DNA and leads to constitutive activation of NF-κB target genes, even without stimulation, whereas most of the p65 remains cytoplasmic. These results indicate that although IκBα, IκBβ and IκBε proteins could be dispensable for cytoplasmic retention of NF-κB, they are essential for preventing NF-κB-dependent gene expression in the basal state. We also show that in the absence of IκBα, IκBβ and IκBε proteins, cytoplasmic retention of NF-κB by other cellular proteins renders the pathway unresponsive to activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Verma, I. M., Stevenson, J. K., Schwarz, E. M., Van Antwerp, D. & Miyamoto, S. Genes Dev. 9, 2723–2735 (1995).
Hayden, M. S. & Ghosh, S. Genes Dev. 18, 2195–2224 (2004).
Karin, M. & Ben-Neriah, Y. Annu. Rev. Immunol. 18, 621–663 (2000).
Bell, S., Matthews, J. R., Jaffray, E. & Hay, R. T. Mol. Cell. Biol. 16, 6477–6485 (1996).
Tran, K., Merika, M. & Thanos, D. Mol. Cell. Biol. 17, 5386–5399 (1997).
Klement, J. F. et al. Mol. Cell. Biol. 16, 2341–2349 (1996).
Beg, A. A., Sha, W. C., Bronson, R. T., Ghosh, S. & Baltimore, D. Nature 376, 167–170 (1995).
Chen, L. F. & Greene, W. C. Nature Rev. Mol. Cell. Biol. 5, 392–401 (2004).
Sachdev, S., Hoffmann, A. & Hannink, M. Mol. Cell. Biol. 18, 2524–2534 (1998).
Tam, W. F., Lee, L. H., Davis, L. & Sen, R. Mol. Cell. Biol. 20, 2269–2284 (2000).
Prigent, M., Barlat, I., Langen, H. & Dargemont, C. J. Biol. Chem. 275, 36441–36449 (2000).
Moorthy, A. K. & Ghosh, G. J. Biol. Chem. 278, 556–566 (2003).
Tergaonkar, V., Bottero, V., Ikawa, M., Li, Q. & Verma, I. M. Mol. Cell. Biol. 23, 8070–8083 (2003).
Tiscornia, G., Singer, O., Ikawa, M. & Verma, I. M. Proc. Natl Acad. Sci. USA 100, 1844–1848 (2003).
Memet, S. et al. J. Immunol. 163, 5994–6005 (1999).
Acknowledgements
We wish to thank A. Hoffmann and D. Baltimore for sharing unpublished information and for insights into this work. We thank C. Rothlin, O. Singer, G. Ghosh, N. Tonnu and B. Coyne for suggestions and help. V.T. is supported by a career development fellowship from the Leukemia and Lymphoma Society. I.M.V. is an American Cancer Society Professor of Molecular Biology and is supported by grants from the NIH, the Wayne and Gladys Valley Foundation, the Larry L. Hillblom Foundation, Inc., the Lebensfeld Foundation, the Merck Research Laboratories and the H.N. and Frances C. Berger Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3 and Supplementary methods (PDF 337 kb)
Rights and permissions
About this article
Cite this article
Tergaonkar, V., Correa, R., Ikawa, M. et al. Distinct roles of IκB proteins in regulating constitutive NF-κB activity. Nat Cell Biol 7, 921–923 (2005). https://doi.org/10.1038/ncb1296
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1296
This article is cited by
-
IKK2/NFkB signaling controls lung resident CD8+ T cell memory during influenza infection
Nature Communications (2023)
-
Hepatocyte Bcl-3 protects from death-receptor mediated apoptosis and subsequent acute liver failure
Cell Death & Disease (2022)
-
IκBα is required for full transcriptional induction of some NFκB-regulated genes in response to TNF in MCF-7 cells
npj Systems Biology and Applications (2021)
-
Autism Spectrum Disorder: Signaling Pathways and Prospective Therapeutic Targets
Cellular and Molecular Neurobiology (2021)
-
Immunomodulatory effects of thalidomide in an experimental brain death liver donor model
Scientific Reports (2021)