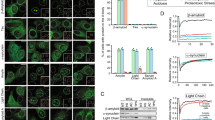
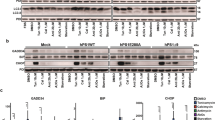
Abstract
Genetic and biochemical evidence have led to the suggestion that presenilins could be the long-searched-for γ-secretase, the proteolytic activity that generates the carboxy terminus of amyloid β-peptides. This activity is also thought to be responsible for the release of the Notch intracellular domain (NICD) from Notch. Here, we report the production of endogenous secreted and intracellular 40- and 42-amino-acid Aβ peptides in mouse fibroblasts deficient in presenilin 1, presenilin 2 or both. We show that the endogenous production of Aβ40 and Aβ42 was not altered by presenilin deficiency. By contrast, inactivating presenilin genes fully abolished NICD production. These data indicate that Aβ and NICD production are distinct catabolic events. Also, even though NICD formation is indeed presenilin dependent, endogenous secreted and intracellular β-amyloid peptides are still generated in absence of presenilins, indicating that there is a γ-secretase activity distinct from presenilins, at least in murine fibroblasts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Checler, F. J. Neurochem. 65, 1431–1444 (1995).
Burdick, D. et al. J. Biol. Chem. 267, 546–554 (1992).
Tanzi, R. E. et al. Neurobiol. Dis. 3, 159–168 (1996).
Li, Y.-M. et al. Proc. Natl. Acad. Sci. USA 97, 6138–6143 (2000).
Li, Y.-M. et al. Nature 405, 689–694 (2000).
Esler, W. P. et al. Nature Cell Biol. 2, 428–434 (2000).
Wolfe, M. S. et al. Nature 398, 513–517 (1999).
Shen, J. et al. Cell 89, 629–639 (1997).
Davis, J. A. et al. Neuron 20, 603–609 (1998).
Donoviel, D. B. et al. Genes Dev. 13, 2801–2810 (1999).
Huppert, S. S. et al. Nature 405, 966–970 (2000).
Schroeter, E. H., Kisslinger, J. A. & Kopan, R. Nature 28, 382–386 (1998).
Herreman, A. et al. Nature Cell Biol. 2, 461–462 (2000).
Zhang, Z. et al. Nature Cell Biol. 2, 463–465 (2000).
Checler, F. J. Neurochem. 76, 1621–1627 (2001).
Wolfe, M. S. J. Neurochem. 76, 1615–1620 (2001).
Petit, A. et al. Nature Cell Biol. 3, 507–511 (2001).
Refolo, L. M. et al. J. Neurochem. 73, 2383–2388 (1999).
Barelli, H. et al. Mol. Med. 3, 695–707 (1997).
Ancolio, C. et al. Proc. Natl. Acad. Sci. USA 96, 4119–4124 (1999).
Chui, D. M. et al. J. Alzheimer's Dis. 3, 231–239 (2001).
Gravina, S. A. et al. J. Biol. Chem. 270, 7013–7016 (1995).
Herreman, A. et al. Proc. Natl. Acad. Sci. USA 96, 11872–11877 (1999).
Xu, H. et al. Nature Med. 4, 447–451 (1998).
Chen, F. et al. J. Biol. Chem. 275, 36794–36802 (2000).
Capell, A. et al. Nature Cell Biol. 2, 205–211 (2000).
L'Hernault, S. W. and Arduengo, P. M. J. Cell. Biol. 119, 55–68 (1992).
De Strooper, B. et al. Nature 391, 387–390 (1998).
De Strooper, B. et al. Nature 398, 518–522 (1999).
Acknowledgements
We thank B. de Strooper (Leuven, Belgium) and P. Saftig (Göttingen, Germany) for providing us with the presenilin-deficient fibroblast cell lines, and R. Kopan for providing us with the mNotchΔE and NICD cDNAs. We are indebted to T. Hartmann and K. Beyreuther for providing WO2 antibodies. We sincerely thank W. Araki and T. Tabira for the gift of anti-presenilin-1 and anti-presenilin-2 antibodies. C. A. C. is supported by a grant from Aventis Pharma. This work was supported by INSERM and by the CNRS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armogida, M., Petit, A., Vincent, B. et al. Endogenous β-amyloid production in presenilin-deficient embryonic mouse fibroblasts. Nat Cell Biol 3, 1030–1033 (2001). https://doi.org/10.1038/ncb1101-1030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1101-1030