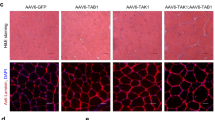
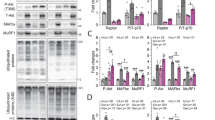
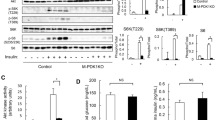
Abstract
Skeletal muscles adapt to changes in their workload by regulating fibre size by unknown mechanisms1,2. The roles of two signalling pathways implicated in muscle hypertrophy on the basis of findings in vitro3,4,5,6, Akt/mTOR (mammalian target of rapamycin) and calcineurin/NFAT (nuclear factor of activated T cells), were investigated in several models of skeletal muscle hypertrophy and atrophy in vivo. The Akt/mTOR pathway was upregulated during hypertrophy and downregulated during muscle atrophy. Furthermore, rapamycin, a selective blocker of mTOR7, blocked hypertrophy in all models tested, without causing atrophy in control muscles. In contrast, the calcineurin pathway was not activated during hypertrophy in vivo, and inhibitors of calcineurin, cyclosporin A and FK506 did not blunt hypertrophy. Finally, genetic activation of the Akt/mTOR pathway was sufficient to cause hypertrophy and prevent atrophy in vivo, whereas genetic blockade of this pathway blocked hypertrophy in vivo. We conclude that the activation of the Akt/mTOR pathway and its downstream targets, p70S6K and PHAS-1/4E-BP1, is requisitely involved in regulating skeletal muscle fibre size, and that activation of the Akt/mTOR pathway can oppose muscle atrophy induced by disuse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carson, J. A. Exercise Sport Science Rev. 25, 301–320 (1997).
Baar, K., Blough, E., Dineen, B. & Esser, K. Exercise Sport Science Rev. 27, 333–379 (1999).
Molkentin, J. D. et al. Cell 93, 215–228 (1998).
Semarian, C. et al. Nature 400, 576–581 (1999).
Musaro, A. et al. Nature 400, 581–585 (1999).
Rommel, C. et al. Nature Cell Biol. 3, 1009–1013 (2001).
Schmeizie, T. & Hall, M. N. Cell 103, 253–262 (2000).
Adams, G. R. & Haddad, G. R. J. Appl. Physiol. 81, 2509–2516 (1996).
Roy, R. R. et al. J. Appl. Physiol. 83, 280–290 (1997).
Naya, F. J. et al. J. Biol. Chem. 275, 4545–4548 (2000).
Murgia, M. et al. Nature Cell Biol. 2, 142–147 (2000).
Terada, N. et al. Proc. Natl Acad. Sci. USA 91, 11477–11481 (1994).
Brunn, G. J. et al. Science 277, 99–101 (1997).
Rhoads, R. E. J. Biol. Chem. 274, 30337–30340 (1999).
Lin, T.-A. et al. Science 266, 653–656 (1994).
Lin, T.-A. & Lawrence, J. C. Jr J. Biol. Chem. 271, 30199–30204 (1996).
Jefferson, L. S., Fabian, J. R. & Kimball, S. R. Int. J. Biochem. Cell Biol. 31, 191–200, (1999).
Welch, G. I., et al. FEBS Lett. 410, 418–422 (1997).
Tung, C. O., Rittenhouse, S. E. & Tsichlis, P. N. Annu. Rev. Biochem. 68, 965–1014 (1999).
Shah, O.J, Anthony, J. C., Kimball, S. R. & Jefferson, L. S. Am. J. Physiol. Endocrinol. Metab. 279, E715–E729 (2000).
Thomason, D. B., Herrick, R. E., Surdyka, D. & Baldwin, K. M. J. Appl. Physiol. 63, 130–137 (1987).
Eves, E. M. et al. Mol. Cell. Biol. 18, 2143–2152 (1998).
Brennan, K. J. & Hardeman, E. C. J. Biol. Chem. 268, 719–725 (1993).
Dunn, S. E., Burns, J. L. & Michel, R. N. J. Biol. Chem. 274, 21908–21912 (1999).
Dunn, S. E., Chin, E. R. & Michel, R. N. J. Cell Biol. 151, 663–672 (2000).
Musaro, A. et al. Nature Genet. 27, 195–200 (2001).
Roy, R. R., Monke, S. R., Allen, D. L. & Edgerton, V. R. J. Appl. Physiol. 87, 634–642 (1999).
Rosenblatt, J. D., Yong, D. & Parry, D. J. Muscle Nerve 17, 608–613 (1994).
Wong, T. S. & Booth F. W. J. Appl. Physiol. 69, 1718–1724 (1990).
Lowe, D. A. & Always, S. E. Cell Tiss. Res. 296, 531–539 (1999).
Baar, K. & Esser, K. Am. J. Physiol. Cell 45, C120–C127 (1999).
Montagne, J. et al. Science 285, 2126–2129 (1999).
Weinkove, D. & Leever, S. J. Curr. Opin. Genet. Dev. 10, 75–80 (2000).
Shima, H. et al. EMBO J. 17, 6649–6659 (1998).
Shioi, T. et al. EMBO J. 19, 2537–2548 (2000).
Rommel, C. et al. Science 286, 1738–1741 (1999).
Azpiazu, I, Saltiel, A. R., DePaoli-Roach, A. A. & Lawrence, J. C. Jr J. Biol. Chem. 271, 5033–5039 (1996).
Acknowledgements
We thank L. S. Schleifer and P. R. Vagelos and the rest of the Regeneron community for their support, particularly E. Burrows for graphics work and C. Rommel for insightful discussions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bodine, S., Stitt, T., Gonzalez, M. et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3, 1014–1019 (2001). https://doi.org/10.1038/ncb1101-1014
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1101-1014
This article is cited by
-
Gromwell ameliorates glucocorticoid-induced muscle atrophy through the regulation of Akt/mTOR pathway
Chinese Medicine (2024)
-
Differential expression of miRNAs associated with pectoral myopathies in young broilers: insights from a comparative transcriptome analysis
BMC Genomics (2024)
-
Effect of Zinc Amino Acid Complexes on Growth Performance, Tissue Zinc Concentration, and Muscle Development of Broilers
Biological Trace Element Research (2024)
-
Insulin signaling in skeletal muscle during inflammation and/or immobilisation
Intensive Care Medicine Experimental (2023)
-
Causal relationship between insulin resistance and sarcopenia
Diabetology & Metabolic Syndrome (2023)