Abstract
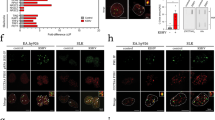
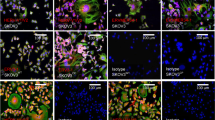
The importance of herpes simplex viruses (HSV) as human pathogens and the emerging prospect of using mutant derivatives of HSV-1 as potential anti-cancer therapeutics have necessitated a thorough investigation into the molecular basis of host-cell permissiveness to HSV. Here we show that NIH-3T3 cells transformed with the oncogenes v-erbB, activated sos or activated ras become significantly more permissive to HSV-1. Inhibitors of the Ras signalling pathway, such as farnesyl transferase inhibitor 1 and PD98059, effectively suppressed HSV-1 infection of ras-transformed cells. Enhanced permissiveness of the transformed cells was linked to the inhibition of virus-induced activation (phosphorylation) of the double-stranded RNA-activated protein kinase (PKR), thereby allowing viral transcripts to be translated in these cells. An HSV-1-derived oncolytic mutant, R3616, was also found to infect preferentially both transformed cells and PKR−/− (but not PKR+/+) mouse embryo fibroblasts. These observations suggest that HSV-1 specifically targets cells with an activated Ras signalling pathway, and have important ramifications in the use of engineered HSV in cancer therapy, the development of strategies against HSV infections, and the controversial role of HSV in human cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Whitley, R. J., Kimberlin, D. W. & Roizman, B. Clin. Infect. Dis. 26, 541–555 (1998).
Markert, J. M. et al. Rev. Med. Virol. 10, 17–30 (2000).
Andreansky, S. S. et al. Cancer Res. 57, 1502–1509 (1997).
Yazaki, T., Manz, H. J., Rabkin, S. D. & Martuza, R. L. Cancer Res. 55, 4752–4756 (1995).
Oyama, M. et al. Hum. Gene Ther. 10, 1683–1693 (2000).
Coukos, G. et al. Cancer Gene Ther. 7, 275–283 (2000).
Sundaresan, P., Hunter, W. D., Martuza, R. L. & Rabkin, S. D. J. Virol. 74, 3832–3841 (2000).
Markert, J. M. et al. Gene Ther. 7, 867–874 (2000).
Shields, J. M., Pruitt, K., McFall, A., Shaub, A. & Der C. J. Trends Cell Biol. 10, 147–154 (2000).
Gibbs, J. B. et al. Curr. Opin. Chem. Biol. 1, 197–203 (1997).
Garrington, T. P. & Johnson, G. L. Curr. Opin. Cell Biol. 11, 211–218 (1999).
Roovers, K. & Assoian, R. K. BioEssays 22, 818–826 (2000).
Dudley, D. T., Pang, L., Decker, S. J., Bridges, A. J. & Saltiel, A. R. Proc. Natl Acad. Sci. USA 92, 7686–7689 (1995).
Cuenda, A. et al. FEBS Lett. 364, 229–233 (1995).
Dong, Z., Huang, C. & Ma, W. Y. Anticancer Res. 19, 3743–3747 (1999).
Nakamura, I. et al. J. Cell Physiol. 172, 230–239 (1997).
White, M. A. et al. Cell 24, 533–541 (1995).
Khosravi-Far, R. et al. Mol. Cell Biol. 16, 3923–3933 (1996).
Webb, C. P., Van Aelst, L., Wigler, M. H. & Woude, G. F Proc. Natl Acad. Sci. USA 21, 8773–8778 (1998).
Roizman, B. & Sears. A. E. in Fields Virology (eds Fields, B. N., Knipe, D. M. & Howley, P. M.) 2231–2295 (Lippincott-Raven, Philadelphia, 1996).
Williams, B. R. Oncogene 18, 6112–6120 (1999).
Clemens, M. J. & Elia, A. J. Interferon Cytokine Res. 17, 503–524 (1997).
Andreansky, S. S. et al. Proc. Natl Acad. Sci. USA 93, 11313–11318 (1996).
He, B., Gross, M. & Roizman, B. Proc. Natl Acad. Sci. USA 94, 843–848 (1997).
Slamon, D. J. & Cline, M. J. Proc. Natl Acad. Sci. USA 81, 7141–7155 (1984).
Strong, J. E., Coffey, M. C., Tang, D., Sabinin, P. & Lee, P. W. K. EMBO J. 17, 3351–3362 (1998).
Coffey, M. C., Strong, J. E., Forsyth, P. A. & Lee, P. W. K. Science 282, 1332–1334 (1998).
Jones, C. Clin. Microbiol. Rev. 8, 549–556 (1995).
Helbing, C. C., Veillette, C., Riabowol, K., Johnston, R. N. & Garkavtsev, I. Cancer Res. 57, 1255–1258 (1997).
Chou, J., Kern, E. R., Whitley, R. J. & Roizman, B. Science 250, 1262–1266 (1990).
Acknowledgements
We thank B. Roizman for the HSV-1 (strain F) and mutant R3616; D. Faller for the NIH-3T3 and H-ras-transformed cells; M. Karin for sos-transformed cells (TNIH#5); H.-J. Kung for THC-11 cells; B. Williams for the PKR−/− and PKR+/+ mouse embryo fibroblasts; R. N. Johnston for NIH3T3 c-myc cells; C. P. Webb for Ras effector domain mutant cell lines; P. Olivo for the anti-ICP8 antibody; K. M. Lee and K. Fonseca for assistance with immunofluorescence studies; and W. Yong, F. Yong, M. Schultz and D. Bazett-Jones for assistance with microscopy. This work was supported by the Canadian Institutes of Health Research (P.W.K.L.). F.F. is a recipient of a Studentship from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farassati, F., Yang, AD. & Lee, P. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol 3, 745–750 (2001). https://doi.org/10.1038/35087061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35087061
This article is cited by
-
Implications of immune cells in oncolytic herpes simplex virotherapy for glioma
Brain Tumor Pathology (2022)
-
Lonidamine potentiates the oncolytic efficiency of M1 virus independent of hexokinase 2 but via inhibition of antiviral immunity
Cancer Cell International (2020)
-
MG132 exerts anti-viral activity against HSV-1 by overcoming virus-mediated suppression of the ERK signaling pathway
Scientific Reports (2020)
-
Reovirus: Friend and Foe
Current Clinical Microbiology Reports (2019)
-
Enhanced Sensitivity of Patient-Derived Pediatric High-Grade Brain Tumor Xenografts to Oncolytic HSV-1 Virotherapy Correlates with Nectin-1 Expression
Scientific Reports (2018)