Abstract
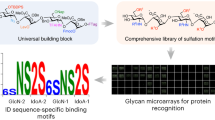
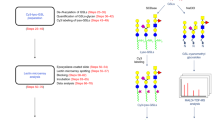
We describe microarrays of oligosaccharides as neoglycolipids and their robust display on nitrocellulose. The arrays are sourced from glycoproteins, glycolipids, proteoglycans, polysaccharides, whole organs, or from chemically synthesized oligosaccharides. We show that carbohydrate-recognizing proteins single out their ligands not only in arrays of homogeneous oligosaccharides but also in arrays of heterogeneous oligosaccharides. Initial applications have revealed new findings, including: (i) among O-glycans in brain, a relative abundance of the Lewisx sequence based on N-acetyllactosamine recognized by anti-L5, and a paucity of the Lewisx sequence based on poly-N-acetyllactosamine recognized by anti-SSEA-1; (ii) insights into chondroitin sulfate oligosaccharides recognized by an antiserum and an antibody (CS-56) to chondroitin sulfates; and (iii) binding of the cytokine interferon-γ (IFN-γ) and the chemokine RANTES to sulfated sequences such as HNK-1, sulfo-Lewisx, and sulfo-Lewisa, in addition to glycosaminoglycans. The approach opens the way for discovering new carbohydrate-recognizing proteins in the proteome and for mapping the repertoire of carbohydrate recognition structures in the glycome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Helenius, A. & Aebi, M. Intracellular functions of N-linked glycans. Science 291, 2364–2369 (2001).
Feizi, T. Progress in deciphering the information content of the 'glycome'—a crescendo in the closing years of the millennium. Glycoconj. J. 17, 553–565 (2000).
Crocker, P.R. & Varki, A. Siglecs in the immune system. Immunology 103, 137–145 (2001).
Karlsson, K.A. Meaning and therapeutic potential of microbial recognition of host glycoconjugates. Mol. Microbiol. 29, 1–11 (1998).
Kiessling, L.L. & Cairo, C.W. Hitting the sweet spot. Nat. Biotechnol. 20, 234–235 (2002).
Wang, D., Liu, S., Trummer, B.J., Deng, C., & Wang, A. Carbohydrate microarrays for the recognition of cross-reactive molecular markers of microbes and host cells. Nat. Biotechnol. 20, 275–281 (2002).
Houseman, B.T. & Mrksich, M. Carbohydrate arrays for the evaluation of protein binding and enzymatic modification. Chem. Biol. 9, 443–454 (2002).
Tang, P.W., Gooi, H.C., Hardy, M., Lee, Y.C. & Feizi, T. Novel approach to the study of the antigenicities and receptor functions of carbohydrate chains of glycoproteins. Biochem. Biophys. Res. Commun. 132, 474–480 (1985).
Stoll, M.S., Mizuochi, T., Childs, R.A. & Feizi, T. Improved procedure for the construction of neoglycolipids having antigenic and lectin-binding activities from reducing oligosaccharides. Biochem. J. 256, 661–664 (1988).
Feizi, T., Stoll, M.S., Yuen, C.-T., Chai, W. & Lawson, A.M. Neoglycolipids: probes of oligosaccharide structure, antigenicity and function. Methods Enzymol. 230, 484–519 (1994).
Stoll, M.S. et al. Fluorescent neoglycolipids: improved probes for oligosaccharide ligand discovery. Eur. J Biochem. 267, 1795–1804 (2000).
Feizi, T. in Carbohydrate Bioengineering: Interdisciplinary Approaches (eds Teeri, T.T., Svensson, B., Gilbert, H.J. & Feizi, T.) 186–193 (The Royal Society of Chemistry, London, UK, 2002).
Yuen, C.-T. et al. Novel sulfated ligands for the cell adhesion molecule E-selectin revealed by the neoglycolipid technology among O-linked oligosaccharides on an ovarian cystadenoma glycoprotein. Biochemistry 31, 9126–9131 (1992).
Chai, W., Feizi, T., Yuen, C.-T. & Lawson, A.M. Nonreductive release of O-linked oligosaccharides from mucin glycoproteins for structure/function assignments as neoglycolipids: application in the detection of novel ligands for E-selectin. Glycobiology 7, 861–872 (1997).
Yuen, C.-T. et al. Brain contains HNK-1 immunoreactive O-glycans of the sulfoglucuronyl lactosamine series that terminate in 2-linked or 2,6-linked hexose (mannose). J. Biol. Chem. 272, 8924–8931 (1997).
Chai, W. et al. High prevalence of 2-mono- and 2,6-di-substituted Manol-terminating sequences among O-glycans released from brain glycopeptides by reductive alkaline hydrolysis. Eur. J. Biochem. 263, 879–888 (1999).
Chai, W., Yuen, C.-T., Feizi, T. & Lawson, A.M. Core-branching pattern and sequence analysis of mannitol-terminating oligosaccharides by neoglycolipid technology. Anal. Biochem. 270, 314–322 (1999).
Leteux, C., Chai, W., Nagai, K., Lawson, A.M. & and Feizi, T. 10E4 Antigen of scrapie lesions contains an unusual nonsulfated heparan motif. J. Biol. Chem. 276, 12539–12545 (2001).
Chou, D.K.H. et al. Structure of sulfated glucuronyl glycolipids in the nervous system reacting with HNK-1 antibody and some IgM paraproteins in neuropathy. J. Biol. Chem. 261, 11717–11725 (1986).
Gooi, H.C. et al. Stage specific embryonic antigen SSEA-1 involves α1-3 fucosylated type 2 blood group chains. Nature 292, 156–158 (1981).
Streit, A. et al. The Lex, carbohydrate sequence is recognized by antibody to L5, a functional antigen in early neural development. J. Neurochem. 66, 834–844 (1996).
Brown, A. et al. A monoclonal antibody against human colonic adenoma recognizes difucosylated Type-2 blood group chains. Biosci. Reps. 3, 163–170 (1983).
Avnur, Z. & Geiger, B. Immunocytochemical localization of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibody. Cell 38, 811–822 (1984).
Feizi, T. in Mammalian Carbohydrate Recognition Systems. Results and Problems in Cell Differentiation, Vol. 33 (ed. Crocker, P.R.) 201–223 (Springer-Verlag, Berlin Heidelberg, 2001).
Hurt-Camejo, E. et al. CD44, a cell surface chondroitin sulfate proteoglycan, mediates binding of interferon-γ and some of its biological effects on human vascular smooth muscle cells. J. Biol. Chem. 274, 18957–18964 (1999).
Hirose, J., Kawashima, H., Yoshie, O., Tashiro, K. & Miyasaka, M. Versican interacts with chemokines and modulates cellular responses. J. Biol. Chem. 276, 5228–5234 (2001).
Hounsell, E.F., Gooi, H.C. & Feizi, T. The monoclonal antibody anti-SSEA-1 discriminates between fucosylated Type 1 and Type 2 blood group chains. FEBS Lett. 131, 279–282 (1981).
Liang, R. et al. Parallel synthesis and screening of a solid phase carbohydrate library. Science 274, 1520–1522 (1996).
Charych, D.H., Nagy, J.O., Spevak, W. & Bednarski, M.D. Direct colorimetric detection of a receptor–ligand interaction by a polymerized bilayer assembly. Science 261, 585–588 (1993).
Crocker, P.R. & Feizi, T. Carbohydrate recognition systems: functional triads in cell–cell interactions. Curr. Opin. Struct. Biol. 6, 679–691 (1996).
Ghosh, P., Bachhawat, B.K. & Surolia, A. Synthetic glycolipids: interaction with galactose-binding lectin and hepatic cells. Arch. Biochem. Biophys. 206, 454–457 (1981).
Surolia, A., Bachhawat, B.K. & Podder, S.K. Interaction between lectin from Ricinus communis and liposomes containing gangliosides. Nature 257, 802–804 (1975).
Galustian, C. et al. Synergistic interactions of the two classes of ligand, sialyl-Lewisa/x fuco-oligosaccharides and short sulpho-motifs, with the P- and L- selectins: implications for therapeutic inhibitor designs. Immunology 105, 350–359 (2002).
Srinivas, O., Mitra, N., Surolia, A. & Jayaraman, N. Photoswitchable multivalent sugar ligands: synthesis, isomerization, and lectin binding studies of azobenzene-glycopyranoside derivatives. J. Am. Chem. Soc. 124, 2124–2125 (2002).
Galustian, C. et al. Valency dependent patterns of reactivity of human L-selectin towards sialyl and sulfated oligosaccharides of Lea and Lex types: relevance to anti-adhesion therapeutics. Biochemistry 36, 5260–5266 (1997).
Stoll, M.S., Hounsell, E.F., Lawson, A.M., Chai, W. & Feizi, T. Microscale sequencing of O-linked oligosaccharides using mild periodate oxidation of alditols, coupling to phospholipid and TLC-MS analysis of the resulting neoglycolipids. Eur. J. Biochem. 189, 499–507 (1990).
Chai, W., Stoll, M.S., Cashmore, G.C. & Lawson, A.M. Specificity of mild periodate oxidation of oligosaccharide-alditol relevance to the analysis of the core-branching pattern of O-linked glycoprotein oligosaccharides. Carbohydr. Res. 239, 107–115 (1993).
Chai, W., Kogelberg, H. & Lawson, A.M. Generation and structural characterization of a range of unmodified chondroitin sulfate oligosaccharide fragments. Anal. Biochem. 237, 88–102 (1996).
Galustian, C. et al. L-selectin interactions with novel mono- and multisulfated Lewisx sequences in comparison with the potent ligand 3′-sulfated Lewisa. J. Biol. Chem. 274, 18213–18217 (1999).
Loveless, R.W., Floyd-O'Sullivan, G., Raynes, J.G. & Feizi, T. Human serum amyloid P is a multispecific adhesive protein whose ligands include 6-phosphorylated mannose and the 3-sulphated saccharides galactose, N-acetylgalactosamine and glucuronic acid. EMBO J. 11, 813–819 (1992).
Hasegawa, A., Ando, T., Kameyama, A. & Kiso, M. Stereocontrolled synthesis of sialyl Lewis X ceramide consisting of pentasaccharide recognized selectin family. Carbohydr. Res. 230, c1–c5 (1992).
Lubineau, A., Le-Gallic, J. & Lemoine, R. First synthesis of the 3′-sulfated Lewis(a) pentasaccharide, the most potent human E-selectin ligand so far. Bioorg. Med. Chem. 2, 1143–1151 (1994).
Lubineau, A., Auge, C., Le Goff, N. & Le Narvor, C. Chemoenzymatic synthesis of a 3IV,6III-disulfated Lewis(x) pentasaccharide, a candidate ligand for human L-selectin. Carbohydr. Res. 305, 501–509 (1997).
Lubineau, A., Alais, J. & Lemoine, R. Synthesis of 3′- and 6'-monosulfated and 3',6'-disulfated Lewis x pentasaccharide, candidate ligands for human L-selectin. J. Carbohydr. Chem. 19, 151–169 (2000).
Komba, S., Ishida, H., Kiso, M. & Hasegawa, A. Synthesis and biological activities of three sulfated sialyl Le(x) ganglioside analogues for clarifying the real carbohydrate ligand structure of L-selection. Bioorg. Med. Chem. 4, 1833–1847 (1996).
Galustian, C. et al. Sialyl-Lewisx sequence 6-O-sulfated at N-acetylglucosamine rather than at galactose is the preferred ligand for L-selectin and de-N-acetylation of the sialic acid enhances the binding strength. Biochem. Biophys. Res. Commun. 240, 748–751 (1997).
Isogai, Y., Kawase, T., Ishida, H., Kiso, M. & Hasegawa, A. Total synthesis of sulfated glucuronyl paraglobosides. J. Carbohydr. Chem. 15, 1001–1023 (1996).
Chai, W., Cashmore, G.C., Carruthers, R.A., Stoll, M.S. & Lawson, A.M. Optimal procedure for combined high-performance thin-layer chromatography/high-sensitivity liquid secondary ion mass spectrometry. Biol. Mass Spectrom. 20, 169–178 (1991).
Bertolotto, A., Palmucci, L., Gagliano, A., Mongini, T. & Tarone, G. Immunohistochemical localization of chondroitin sulfate in normal and pathological human muscle. J. Neurol. Sci. 73, 233–244 (1986).
Smith, P.L. et al. Expression of the α(1-3)fucosyltransferase Fuc-TVII in lymphoid aggregate high endothelial venules correlates with expression of L-selectin ligands. J. Biol. Chem. 271, 8250–8259 (1996).
Acknowledgements
This work is supported by a program grant G9601454 from the UK Medical Research Council. We thank D. Wang and W. Loveless for stimulating discussions, C. Herbert for technical support, and C. Kelly for assistance in compiling the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fukui, S., Feizi, T., Galustian, C. et al. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nat Biotechnol 20, 1011–1017 (2002). https://doi.org/10.1038/nbt735
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt735
This article is cited by
-
Genetically encoded multivalent liquid glycan array displayed on M13 bacteriophage
Nature Chemical Biology (2021)
-
Surface plasmon resonance imaging coupled to on-chip mass spectrometry: a new tool to probe protein-GAG interactions
Analytical and Bioanalytical Chemistry (2020)
-
130 years of Plant Lectin Research
Glycoconjugate Journal (2020)
-
Enhanced Neuronal Survival and Neurite Outgrowth Triggered by Novel Small Organic Compounds Mimicking the LewisX Glycan
Molecular Neurobiology (2018)