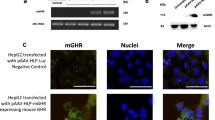
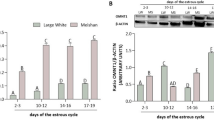
Abstract
Ectopic expression of a new serum protease-resistant porcine growth hormone–releasing hormone, directed by an injectable muscle-specific synthetic promoter plasmid vector (pSP-HV-GHRH), elicits growth in pigs. A single 10 mg intramuscular injection of pSP-HV-GHRH DNA followed by electroporation in three-week-old piglets elevated serum GHRH levels by twofold to fourfold, enhanced growth hormone secretion, and increased serum insulin-like growth factor-I by threefold to sixfold over control pigs. After 65 days the average body weight of the pigs injected with pSP-HV-GHRH was ~37% greater than the placebo-injected controls and resulted in a significant reduction in serum urea concentration, indicating a decrease in amino acid catabolism. Evaluation of body composition indicated a uniform increase in mass, with no organomegaly or associated pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Frohman, L.A., Downs, T.R., Chomczynski, P. Regulation of growth hormone secretion. Front. Neuroendocrinol.. 13, 344–405 (1992).
Thorner, M.O., Chapman, I.M., Gaylinn, B.D., Pezzoli, S.S. & Hartman, M.L. Growth hormone-releasing hormone and growth hormone releasing peptide as therapeutic agents to enhance growth hormone secretion in disease and aging. Recent. Prog. Horm. Res.. 52, 215–244 ( 1997).
Parks, J.S., Pfaffle, R.W., Brown, M.R., Abdul-Latif, H. & Meacham, L.R. in Growth hormone deficiency (ed. Weintraub, B.D.) 473–490 (Raven Press, New York; 1995).
Jacobs, P.A. et al. A cytogenetic and molecular reappraisal of a series of patients with Turner's syndrome. Ann. Hum. Genet. 54, 209 –223 (1990).
Tanaka, H., Kubo, T., Yamate, T., Ono, T., Kanzaki, S. & Seino, Y. Effect of growth hormone therapy in children with achondroplasia: growth pattern, hypothalamic-pituitary function, and genotype. Eur. J. Endocrinol.. 138, 275–280 (1998).
Savage, M.O., Beattie, R.M., Camacho-Hubner, C., Walker-Smith, J.A. & Sanderson, I.R. Growth in Crohn's disease. Acta Paediatr. Scand. Suppl. 428, 89–92 (1999).
Albanese, A. & Stanhope, R. GH treatment induces sustained catch-up growth in children with intrauterine growth retardation: 7-year results. Horm. Res. 48, 173–177 (1997).
Benfield, M.R. & Kohaut, E.C. Growth hormone is safe in children after renal transplantation. J. Pediatr. 131, S28–S31 (1997).
Gesundheit, N. & Alexander, J.K. in Endocrine therapy with recombinant hormones and growth factors (ed. Weintraub, B.D.) 491– 507 (Raven Press, New York; 1995).
Veldhuis, J.D., Iranmanesh, A. & Weltman, A. Elements in the pathophysiology of diminished growth hormone (GH) secretion in aging humans. Endocrine. 7 , 41–48 (1997).
Heptulla, R.A. et al. Decreased insulin sensitivity and compensatory hyperinsulinemia after hormone treatment in children with short stature. J. Clin. Endocrinol. & Metab. 82, 3234– 3238 (1997).
Watkins, S.L. Bone disease in patients receiving growth hormone. Kidney Int. Suppl. 53, S126–S127 ( 1996).
Lapierre, H. et al. Effect of human growth hormone-releasing factor and(or) thyrotropin-releasing factor on growth, carcass composition, diet digestibility, nutrient balance, and plasma constituents in dairy calves. J. Anim. Sci. 69, 587–598 (1991).
Etherton, T.D. & Bauman, D.E. Biology of somatotropin in growth and lactation of domestic animals. Physiol. Rev. 78, 745–761 (1998).
Etherton, T.D. et al. Stimulation of pig growth performance by porcine growth hormone and growth hormone-releasing factor. J. Anim. Sci. 63, 1389–1399 (1986).
Corpas, E., Harman, S.M., Pineyro, M.A., Roberson, R. & Blackman, M.R. Continuous subcutaneous infusions of growth hormone (GH) releasing hormone 1-44 for 14 days increase GH and insulin-like growth factor-I levels in old men. J. Clin. Endocrinol. Metab. 76, 134–138 ( 1993).
Thorner, M.O. et al. Extrahypothalamic growth-hormone-releasing factor (GRF) secretion is a rare cause of acromegaly: plasma GRF levels in 177 acromegalic patients J. Clin. Endocrinol. Metab. 59, 846– 849 (1984).
Muramatsu, T., Nakamura, A. & Park, H.M. In vivo electroporation: a powerful and convenient means of nonviral gene transfer to tissues of living animals. Int. J. Mol. Med.. 1, 55–62 (1998).
Davis, H.L., Whalen, R.G. & Demeneix, B.A. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum. Gene Ther. 4, 151–159 (1993).
Tripathy, S.K. et al. Long-term expression of erythropoietin in the systemic circulation of mice after intramuscular injection of a plasmid DNA vector. Proc. Natl. Acad. Sci. USA. 93, 10876– 10880 (1996).
Li, X., Eastman, E.M., Schwartz, R.J. & Draghia-Akli, R. Synthetic muscle promoters: activities exceeding naturally occurring regulatory sequences. Nat. Biotechnol. 17, 241– 245. (1999).
Mir, L.M. et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. USA. 96, 4262– 4267 (1999).
Frohman, L.A. et al. Metabolic clearance and plasma disappearance rates of human pancreatic tumor growth hormone releasing factor in man. J. Clin. Invest.. 73, 1304–1311 ( 1984).
Su, C.M. et al. In vitro stability of growth hormone releasing factor (GRF) analogs in porcine plasma. Horm. Metab. Res. 23, 15– 21 (1991).
Campbell, R.M. et al. GRF analogs and fragments: correlation between receptor binding, activity and structure. Peptides. 12, 569 –574 (1991).
Kubiak, T.M., Kelly, C.R. & Krabill, L.F. In vitro metabolic degradation of a bovine growth hormone-releasing factor analog Leu27-bGRF(1-29)NH2 in bovine and porcine plasma. Correlation with plasma dipeptidylpeptidase activity. Drug Metab. Dispos. 17, 393–397 (1989).
Martin, R.A., Cleary, D.L., Guido, D.M., Zurcher-Neely, H.A., Kubiak, T.M. Dipeptidyl peptidase IV (DPP-IV) from pig kidney cleaves analogs of bovine growth hormone-releasing factor (bGRF) modified at position 2 with Ser, Thr or Val. Extended DPP-IV substrate specificity? Biochim. Biophys. Acta. 1164, 252–260 (1993).
Aihara, H. & Miyazaki, J. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16, 867– 870 (1998).
Ellis, K.J. & Eastman J.D. In vivo methods, models, and assessment. Basic Life Sciences. Vol. 60 (ed. Chu, E.H.Y.) 153–397 (Plenum Press, New York; 1993 ).
Frohman, L.A., Downs, T.R., Heimer, E.P. & Felix, A.M. Dipeptidylpeptidase IV and trypsin-like enzymatic degradation of human growth hormone-releasing hormone in plasma. J. Clin. Invest. 83, 1533–1540 (1989).
Draghia-Akli, R., Li, X.G. & Schwartz, R.J. Enhanced growth by ectopic expression of growth hormone releasing hormone using an injectable myogenic vector. Nat. Biotechnol.. 15, 1285–1289 ( 1997).
Bergsma, D.J., Grichnik, J.M., Gossett, L.M. & Schwartz, R.J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol. Cell. Biol. 6, 2462 –2475 (1986).
Tanner, J.W., Davis, S.K., McArthur, N.H., French, J.T. & Welsh, T.H. Jr., Modulation of growth hormone (GH) secretion and GH mRNA levels by GH-releasing factor, somatostatin and secretagogues in cultured bovine adenohypophysial cells. J. Endocrinol. 125, 109–115 (1990).
Acknowledgements
We thank Jim Cunningham, Frankie Biggs, Craig Stubblefield, and MichaelStubblefield for excellent care of the animals; Dr. Harry Mersmann, RomanShipaylo, and Dr. Ken Ellis for the body composition measurements; CharlesMcDonald and Dr. Richard Cook for the synthesis of the HV-GHRH polypeptide;Jana Peters for the GH assays; and Dr. Craig Delaughter for carefully reviewing thismanuscript. We acknowledge support for this study from Applied VeterinarySystems Inc. (Houston, TX), The Texas Advanced Technology Program, and theNational Space Biological Research Institute (NASA), the US Department ofAgriculture, Agricultural Research Service under Cooperative Agreement number58-6250-6001. The contents of this publication do not necessarily reflect the viewsor policies of the US Department of Agriculture nor does mention of trade names orcommercial products or organizations imply endorsements by the US government.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Draghia-Akli, R., Fiorotto, M., Hill, L. et al. Myogenic expression of an injectable protease-resistant growth hormone–releasing hormone augments long-term growth in pigs. Nat Biotechnol 17, 1179–1183 (1999). https://doi.org/10.1038/70718
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/70718
This article is cited by
-
Efficient base editing by RNA-guided cytidine base editors (CBEs) in pigs
Cellular and Molecular Life Sciences (2020)
-
Efficient Generation of Orthologous Point Mutations in Pigs via CRISPR-assisted ssODN-mediated Homology-directed Repair
Molecular Therapy - Nucleic Acids (2016)
-
Adiponectin gene therapy ameliorates high-fat, high-sucrose diet-induced metabolic perturbations in mice
Nutrition & Diabetes (2012)
-
A Comparison of the Growth Responses Following Intramuscular GHRH Plasmid Administration Versus Daily Growth Hormone Injections in Young Pigs
Molecular Therapy (2010)
-
Construction and analysis of compact muscle-specific promoters for AAV vectors
Gene Therapy (2008)