Abstract
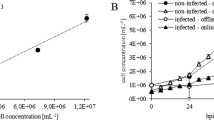
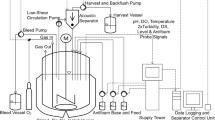
We demonstrate that insect cells can be grown in an agitated, sparged bioreactor and an airlift bioreactor with a growth rate and maximum cell density comparable to those obtained in spinner flasks oxygenated by surface diffusion. Recombinant protein synthesis by cells infected with a genetically-modified baculovirus in the sparged bioreactor is also demonstrated. The nonionic surfactant Pluronic® F-68 was responsible for the protection of insect cells from the adverse effects of sparging. The mechanism of Pluronic® F-68 protection in a sparged environment is discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Luckow, V.A. and Summers, M.D. 1988. Trends in the development of baculovirus expression vectors. Bio/Technology 6:47–55.
Brown, M. and Faulkner, P. 1975. Factors affecting the yield of virus in a cloned cell line of Trichoplusia ni infected with a nuclear polyhedrosis virus. J. Invert. Path. 26:251–257.
Hink, W.F. 1982. Production of Autographa California nuclear polyhedrosis virus in cells from large-scale suspension cultures, 493–506. In: Microbial and Viral Pesticides. Kurstak, E. (Ed.), Marcel Dekker, Inc.
Vaughn, J.L. 1976. Production of NPV in large-scale cultures. J. Invert. Path. 28:233–237.
Weiss, S.A., Peplow, D., Smith, G.C., Vaughn, J.L., and Dougherty, E. 1985. Biotechnical aspects of a large-scale process for insect cells and baculoviruses, 1–16. In: Techniques in the Life Sciences: Techniques in Setting up and Maintenance of Tissue and Cell Cultures, Vol. C110. Kurstak, E. (Ed.), Elsevier Scientific Publishing Co.
Weiss, S.A. and Vaughn, J.L. 1986. Cell culture methods for large-scale propagation of baculoviruses, 63–87. In: The Biology of Baculoviruses, Vol. II. Granados, R. R. and Federici, B. A. (Eds.), CRC Press, Inc.
Aunins, J.G., Croughan, M.S., Wang, D.I.C., and Goldstein, J.M. 1986. Engineering developments in homogeneous culture of animal cells: oxygenation of reactors and scaleup, 699–723. In: Biotechnology and Bioengineering Symposium No. 17. Scott, C. D. (Ed.), John Wiley and Sons, Inc.
Spier, R.E. and Griffiths, B. 1984. An examination of the data and concepts germane to the oxygenation of cultured animal cells. Develop. Biol. Standard 55:81–92.
Miltenburger, H.G. and David, P. 1980. Mass production of insect cells in suspension. Develop. Biol. Standard. 46:183–186.
Tramper, J., Williams, J.B., and Joustra, D. 1986. Shear sensitivity of insect cells in suspension. Enzyme Microb. Technol. 8:33–36.
Handa, A., Emery, A.N., and Spier, R.E. 1987. On the evaluation of gas-liquid interfacial effects on hybridoma viability in bubble column bioreactors. Develop. Biol. Standard. 66:241–252.
Handa, A., Emery, A.N., and Spier, R.E. 1987. Effect of gas-liquid interfaces on the growth of suspended mammalian cells: mechanisms of cell damage by bubbles. Presented at the 8th Meeting of European Society for Animal Cell Technology, Israel.
Tramper, J., Joustra, D., and Vlak, J.M. 1987. Bioreactor design for growth of shear-sensitive insect cells, 125–136. In: Plant and Animal Cells. Webb and Mavituna (Eds.), Ellis Horwood Ltd., Chichester, England.
Tramper, J., Smit, D., Straatman, J., and Vlak, J.M. 1988. Bubble-column design for growth of fragile insect cells. Bioprocess Engineering 3:37–41.
Swim, H.E. and Parker, R.F. 1960. Effect of Pluronic® F-68 on growth of fibroblasts in suspension on rotary shaker. Proc. Soc. Exp. Biol. Med. 103:252–254.
Schmolka, I.R. 1977. A review of block polymer surfactants. J. Amer. Oil Chemists' Soc. 54:110–116.
Reuveny, S., Velez, D., Macmillan, J.D., and Miller, L. 1986. Comparison of cell propagation methods for their effect on monoclonal antibody production in stirred reactors. J. Immunological Methods 86:53–59.
Radlett, P.J., Telling, R.C., Stone, C.J., and Whiteside, J.P. 1971. Improvements in the growth of BHK-21 cells in submerged culture. Applied Microbiology 22:534–537.
Kilburn, D.G. and Webb, F.C. 1968. The cultivation of animal cells at controlled dissolved oxygen partial pressure. Biotechnol. Bioeng. 10:801–814.
Summers, M.D. and Smith, G.E. 1987. A Manual of Methods for Baculovirus Vectors and Insect Cell Culture Procedures. Texas Agricultural Experimental Station Bulletin No. 1555.
Tramper, J. and Vlak, J.M. 1986. Some engineering and economic aspects of continuous cultivation of insect cells for the production of baculoviruses. Biochemical Engineering IV (reprinted from vol. 469, Annals of the New York Academy of Sciences), 279–288.
Pappenheimer, A.M. 1917. Experimental studies upon lymphocytes. I. The reactions of lymphocytes under various experimental conditions. J. Exp. Med. 25:633–650.
Phillips, H.J. 1973. Dye exclusion tests for cell viability, 406–408. In: Tissue Culture: Methods and Applications. Kruse, P. F. and Patterson, M. K. (Eds.), Academic Press.
Volkman, L.E. and Summers, M.D. 1977. Autographa californica nuclear polyhedrosis virus: comparative infectivity of the occluded, alkali-liberated, and nonoccluded forms. J. Invert. Path. 30:102–103.
Mizrahi, A. 1984. Oxygen in human lymphoblastoid cell line cultures and effect of polymers in agitated and aerated cultures. Develop. Biol. Stand. 55:93–102.
Hughes, P.R. and Wood, H.A. 1986. In vivo and in vitro bioassay methods for baculoviruses, 1–30. In: The Biology of Baculoviruses, vol. II. Granados, R. R. and Federici, B. A. (Eds.), CRC Press, Inc.
Miller, J.H. 1972. Assay of β-galactosidase, P. 352–355. In: Experiments in Molecular Genetics. Cold Spring Harbor Laboratory.
Miller, J.H. 1972. Purification of β-galactosidase, 398–404. In: Experiments in Molecular Genetics. Cold Spring Harbor Laboratory.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murhammer, D., Goochee, C. Scaleup of Insect Cell Cultures: Protective Effects of Pluronic F-68. Nat Biotechnol 6, 1411–1418 (1988). https://doi.org/10.1038/nbt1288-1411
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt1288-1411
This article is cited by
-
Impact of Pluronic® F68 on hollow fiber filter-based perfusion culture performance
Bioprocess and Biosystems Engineering (2017)
-
Utilization of pyrolytic substrate by microalga Chlamydomonas reinhardtii: cell membrane property change as a response of the substrate toxicity
Applied Microbiology and Biotechnology (2016)
-
Pluronic F-68: an answer for shoot regeneration recalcitrance in microspore-derived Brassica napus embryos
In Vitro Cellular & Developmental Biology - Plant (2011)