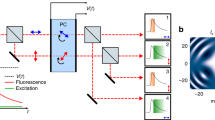
Abstract
Fluorescence microscopy of living cells enables visualization of the dynamics and interactions of intracellular molecules. However, fluorescence live-cell imaging is limited by photobleaching and phototoxicity induced by the excitation light. Here we describe controlled light-exposure microscopy (CLEM), a simple imaging approach that reduces photobleaching and phototoxicity two- to tenfold, depending on the fluorophore distribution in the object. By spatially controlling the light-exposure time, CLEM reduces the excitation-light dose without compromising image quality. We show that CLEM reduces photobleaching sevenfold in tobacco plant cells expressing microtubule-associated GFP-MAP4 and reduces production of reactive oxygen species eightfold and prolongs cell survival sixfold in HeLa cells expressing chromatin-associated H2B-GFP. In addition, CLEM increases the dynamic range of the fluorescence intensity at least twofold.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wright, A., Bubb, W.A., Hawkins, C.L. & Davies, M.J. Singlet oxygen-mediated protein oxidation: evidence for the formation of reactive side chain peroxides on tyrosine residues. Photochem. Photobiol. 76, 35–46 (2002).
Bartosz, G. Oxidative stress in plants. Acta Physiol. Plant. 19, 47–64 (1997).
Foyer, C.H., Lelandais, M. & Kunert, K.J. Photooxidative stress in plants. Physiol. Plant. 92, 696–717 (1994).
Bernas, T., Zarebski, M., Cook, R.R. & Dobrucki, J.W. Minimizing photobleaching during confocal microscopy of fluorescent probes bound to chromatin: role of anoxia and photon flux. J. Microsc. 215, 281–296 (2004).
Song, L., Varma, C.A., Verhoeven, J.W. & Tanke, H.J. Influence of the triplet excited state on the photobleaching kinetics of fluorescein in microscopy. Biophys. J. 70, 2959–2968 (1996).
Song, L., van Gijlswijk, R.P., Young, I.T. & Tanke, H.J. Influence of fluorochrome labeling density on the photobleaching kinetics of fluorescein in microscopy. Cytometry 27, 213–223 (1997).
Vrouenraets, M.B., Visser, G.W., Snow, G.B. & van Dongen, G.A. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 23, 505–522 (2003).
Dixit, R. & Cyr, R. Cell damage and reactive oxygen species production induced by fluorescence microscopy: effect on mitosis and guidelines for non-invasive fluorescence microscopy. Plant J. 36, 280–290 (2003).
Martin, R.M., Leonhardt, H. & Cardoso, M.C. DNA labeling in living cells. Cytometry A 67, 45–52 (2005).
Hsi, R.A., Rosenthal, D.I. & Glatstein, E. Photodynamic therapy in the treatment of cancer: current state of the art. Drugs 57, 725–734 (1999).
Sugden, J.K. Photochemistry of dyes and fluorochromes used in biology and medicine: some physicochemical background and current applications. Biotech. Histochem. 79, 71–90 (2004).
Stephens, D.J. & Allan, V.J. Light microscopy techniques for live cell imaging. Science 300, 82–86 (2003).
Rieder, C.L. & Khodjakov, A. Mitosis through the microscope: advances in seeing inside live dividing cells. Science 300, 91–96 (2003).
Sheppard, C.J.R., Gan, X., Gu, M. & Roy, M. Signal-to-noise in confocal microscopes. in Handbook of Biological Confocal Microscopy, edn. 2 (ed. Pawley, J.B.) 363–372 (Plenum Press, New York, 1995).
Centonze, V. & Pawley, J. Tutorial on practical confocal microscopy and use of the confocal test specimen. in Handbook of Biological Confocal Microscopy, edn. 2 (ed. Pawley, J.B.) 549–569 (Plenum Press, New York, 1995).
Manders, E.M.M. Werkwijze en inrichting voor het vormen van een afbeelding van een object. Patent NL1023440C (filed 16 May 2003, approved 3 January 2005).
Manders, E.M.M. Method and apparatus for shaping an image of an object. Patent application WO2004102249 (2004).
Zwier, J.M., Van Rooij, G.J., Hofstraat, J.W. & Brakenhoff, G.J. Image calibration in fluorescence microscopy. J. Microsc. 216, 15–24 (2004).
Eggeling, C., Widengren, J., Rigler, R. & Seidel, C.A.M. Photobleaching of fluorescent dyes under conditions used for single-molecule detection: evidence of two-step photolysis. Anal. Chem. 70, 2651–2659 (1998).
Molski, A. Statistics of the bleaching number and the bleaching time in single-molecule fluorescence spectroscopy. J. Chem. Phys. 114, 1142–1147 (2001).
Eggeling, C., Volkmer, A. & Seidel, C.A.M. Molecular photobleaching kinetics of rhodamine 6G by one- and two-photon induced confocal fluorescence microscopy. ChemPhysChem 6, 791–804 (2005).
Deschenes, L.A. & Bout, D.A.V. Single molecule photobleaching: increasing photon yield and survival time through suppression of two-step photolysis. Chem. Phys. Lett. 365, 387–395 (2002).
Van Oostveldt, P., Verhaegen, F. & Messens, K. Heterogeneous photobleaching in confocal microscopy caused by differences in refractive index and excitation mode. Cytometry 32, 137–146 (1998).
Rigaut, J.P. & Vassy, J. High-resolution three-dimensional images from confocal scanning laser microscopy. Quantitative study and mathematical correction of the effects from bleaching and fluorescence attenuation in depth. Anal. Quant. Cytol. Histol. 13, 223–232 (1991).
Dhonukshe, P. & Gadella, T.W., Jr. Alteration of microtubule dynamic instability during preprophase band formation revealed by yellow fluorescent protein-CLIP170 microtubule plus-end labeling. Plant Cell 15, 597–611 (2003).
Knight, M.M., Roberts, S.R., Lee, D.A. & Bader, D.L. Live cell imaging using confocal microscopy induces intracellular calcium transients and cell death. Am. J. Physiol. Cell Physiol. 284, C1083–C1089 (2003).
Jou, M.J., Jou, S.B., Chen, H.M., Lin, C.H. & Peng, T.I. Critical role of mitochondrial reactive oxygen species formation in visible laser irradiation-induced apoptosis in rat brain astrocytes (RBA-1). J. Biomed. Sci. 9, 507–516 (2002).
Kanda, T., Sullivan, K.F. & Wahl, G.M. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8, 377–385 (1998).
Lippincott-Schwartz, J. & Patterson, G.H. Development and use of fluorescent protein markers in living cells. Science 300, 87–91 (2003).
Houtsmuller, A.B. Fluorescence recovery after photobleaching: application to nuclear proteins. Adv. Biochem. Eng. Biotechnol. 95, 177–199 (2005).
Houtsmuller, A.B. et al. Action of DNA repair endonuclease ERCC1/XPF in living cells. Science 284, 958–961 (1999).
Acknowledgements
E.M.M.M., R.A.H., C.H.V.O., C.J.F.V.N. and T.W.J.G. were supported by the Amsterdam Genomics Centre (AmGC) of the University of Amsterdam, E.M.M.M. by the Dutch Technology Foundation (STW, ATF-7394); E.M.M.M., R.A.H. and T.W.J.G., by the EU integrated project “Molecular Imaging” (LSHG-CT-2003-503259); and P.B.D. and T.W.J.G., by the Netherlands Organization for Scientific Research (NWO, grant 805-47.012). We thank Merel Adjobo-Hermans, Joachim Goedhart and Bernd Rieger for technical assistance, G.J. Brakenhoff for scientific advice and Nikon Europe for providing microscope equipment.
Author information
Authors and Affiliations
Contributions
R.A.H., experimental data, engineering, computer simulations; C.H.V.O., electronic design and development; T.W.J.G., biofluorescence expertise; P.B.D., plant cell experiments; C.J.F.V.N., scientific and editorial management; E.M.M.M., inventor of CLEM and general team leader.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Reduction of photobleaching and phototoxicity by CLEM depends of fluorophore distribution. (PDF 6740 kb)
Supplementary Video 1
CLEM strongly reduces photobleaching. Left: non-CLEM; Right: CLEM (MOV 1200 kb)
Supplementary Video 2
CLEM strongly reduces phototoxicity. Left: non-CLEM; Right: CLEM (MOV 9713 kb)
Rights and permissions
About this article
Cite this article
Hoebe, R., Van Oven, C., Gadella, T. et al. Controlled light-exposure microscopy reduces photobleaching and phototoxicity in fluorescence live-cell imaging. Nat Biotechnol 25, 249–253 (2007). https://doi.org/10.1038/nbt1278
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1278
This article is cited by
-
Quantitative phase imaging by gradient retardance optical microscopy
Scientific Reports (2024)
-
Artificial confocal microscopy for deep label-free imaging
Nature Photonics (2023)
-
Label-free mid-infrared photothermal live-cell imaging beyond video rate
Light: Science & Applications (2023)
-
Single-shot isotropic differential interference contrast microscopy
Nature Communications (2023)
-
Real-time denoising enables high-sensitivity fluorescence time-lapse imaging beyond the shot-noise limit
Nature Biotechnology (2023)