Abstract
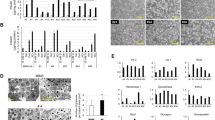
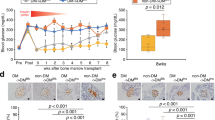
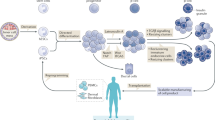
A human pancreatic β-cell line that is functionally equivalent to primary β-cells has not been available. We established a reversibly immortalized human β-cell clone (NAKT-15) by transfection of primary human β-cells with a retroviral vector containing simian virus 40 large T-antigen (SV40T) and human telomerase reverse transcriptase (hTERT) cDNAs flanked by paired loxP recombination targets, which allow deletion of SV40T and TERT by Cre recombinase. Reverted NAKT-15 cells expressed β-cell transcription factors (Isl-1, Pax 6, Nkx 6.1, Pdx-1), prohormone convertases 1/3 and 2, and secretory granule proteins, and secreted insulin in response to glucose, similar to normal human islets. Transplantation of NAKT-15 cells into streptozotocin-induced diabetic severe combined immunodeficiency mice resulted in perfect control of blood glucose within 2 weeks; mice remained normoglycemic for longer than 30 weeks. The establishment of this cell line is one step toward a potential cure of diabetes by transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yoon, J.W. & Jun, H.S. Insulin-dependent diabetes mellitus. in Encyclopedia of Immunology (eds. Roitt, I.M. & Delves, P.J.) 1390–1398, (Academic Press, London, 1998).
Ricordi, C. & Strom, T.B. Clinical islet transplantation: advances and immunological challenges. Nat. Rev. Immunol. 4, 259–268 (2004).
Shapiro, A.M., Nanji, S.A. & Lakey, J.R. Clinical islet transplant: current and future directions towards tolerance. Immunol. Rev. 196, 219–236 (2003).
Moritoh, Y., Yamato, E., Yasui, Y., Miyazaki, S. & Miyazaki, J. Analysis of insulin-producing cells during in vitro differentiation from feeder-free embryonic stem cells. Diabetes 52, 1163–1168 (2003).
Assady, S. et al. Insulin production by human embryonic stem cells. Diabetes 50, 1691–1697 (2001).
Soria, B. et al. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 49, 157–162 (2000).
Korsgren, O., Buhler, L.H. & Groth, C.G. Toward clinical trials of islet xenotransplantation. Xenotransplantation 10, 289–292 (2003).
Beattie, G.M. et al. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes 51, 3435–3439 (2002).
Hayek, A. et al. Growth factor/matrix-induced proliferation of human adult beta-cells. Diabetes 44, 1458–1460 (1995).
Bonner-Weir, S. et al. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA 97, 7999–8004 (2000).
Halvorsen, T.L., Beattie, G.M., Lopez, A.D., Hayek, A. & Levine, F. Accelerated telomere shortening and senescence in human pancreatic islet cells stimulated to divide in vitro. J. Endocrinol. 166, 103–109 (2000).
de la Tour, D. et al. Beta-cell differentiation from a human pancreatic cell line in vitro and in vivo. Mol. Endocrinol. 15, 476–483 (2001).
Matsumura, T. et al. Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation 77, 1357–1365 (2004).
Milo-Landesman, D. et al. Correction of hyperglycemia in diabetic mice transplanted with reversibly immortalized pancreatic beta cells controlled by the tet-on regulatory system. Cell Transplant. 10, 645–650 (2001).
Halvorsen, T.L., Leibowitz, G. & Levine, F. Telomerase activity is sufficient to allow transformed cells to escape from crisis. Mol. Cell. Biol. 19, 1864–1870 (1999).
Fleischer, N. et al. Functional analysis of a conditionally transformed pancreatic beta-cell line. Diabetes 47, 1419–1425 (1998).
Efrat, S., Fusco-DeMane, D., Lemberg, H., al Emran, O. & Wang, X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc. Natl. Acad. Sci. USA 92, 3576–3580 (1995).
Narushima, M. et al. Adenovirus mediated gene transduction of primarily isolated mouse islets. ASAIO J. 50, 586–590 (2004).
Bollheimer, L.C. et al. Insulin-sparing effects of troglitazone in rat pancreatic islets. J. Mol. Endocrinol. 31, 61–69 (2003).
Ohgawara, H., Shikano, T., Fukunaga, K., Yamagishi, M. & Miyazaki, S. Establishment of monolayer culture of pig pancreatic endocrine cells by use of nicotinamide. Diabetes Res. Clin. Pract. 42, 1–8 (1998).
Halban, P.A. et al. The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology 111, 86–94 (1982).
Kobayashi, N. et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science 287, 1258–1262 (2000).
Zalzman, M. et al. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc. Natl. Acad. Sci. USA 100, 7253–7258 (2003).
Shen, L. et al. DNA methylation and environmental exposures in human hepatocellular carcinoma. J. Natl. Cancer Inst. 94, 755–761 (2002).
Mukai, T. & Sekiguchi, M. Gene silencing in phenomena related to DNA repair. Oncogene 21, 9033–9042 (2002).
Bae, S.C. & Choi, J.K. Tumor suppressor activity of RUNX3. Oncogene 23, 4336–4340 (2004).
Giannoukakis, N. & Trucco, M. Current status and prospects for gene and cell therapeutics for type 1 diabetes mellitus. Rev. Endocr. Metab. Disord. 4, 369–380 (2003).
Jun, H.S. & Yoon, J.W. Approaches for the cure of type 1 diabetes by cellular and gene therapy. Curr. Gene Ther. 5, 249–262 (2005).
Shapiro, A.M. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238 (2000).
Linetsky, E. et al. Improved human islet isolation using a new enzyme blend, liberase. Diabetes 46, 1120–1123 (1997).
Ricordi, C., Lacy, P.E. & Scharp, D.W. Automated islet isolation from human pancreas. Diabetes (suppl. 1) 38, 140–142 (1989).
Westerman, K.A. & Leboulch, P. Reversible immortalization of mammalian cells mediated by retroviral transfer and site-specific recombination. Proc. Natl. Acad. Sci. USA 93, 8971–8976 (1996).
Lukowiak, B. et al. Identification and purification of functional human beta-cells by a new specific zinc-fluorescent probe. J. Histochem. Cytochem. 49, 519–528 (2001).
Watanabe, T. et al. Establishment of immortalized human hepatic stellate scavenger cells to develop bioartificial livers. Transplantation 75, 1873–1880 (2003).
Ahlgren, U., Pfaff, S.L., Jessell, T.M., Edlund, T. & Edlund, H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature 385, 257–260 (1997).
Jensen, J., Serup, P., Karlsen, C., Nielsen, T.F. & Madsen, O.D. mRNA profiling of rat islet tumors reveals nkx 6.1 as a beta-cell-specific homeodomain transcription factor. J. Biol. Chem. 271, 18749–18758 (1996).
Jonsson, J., Carlsson, L., Edlund, T. & Edlund, H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371, 606–609 (1994).
Nakabayashi, H., Taketa, K., Miyano, K., Yamane, T. & Sato, J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 42, 3858–3863 (1982).
Ohtani, S. et al. Quantitative analysis of p53-targeted gene expression and visualization of p53 transcriptional activity following intratumoral administration of adenoviral p53 in vivo. Mol. Cancer Ther. 3, 93–100 (2004).
Le Lay, J., Matsuoka, T.A., Henderson, E. & Stein, R. Identification of a novel PDX-1 binding site in the human insulin gene enhancer. J. Biol. Chem. 279, 22228–22235 (2004).
Garson, J.A., van den Berghe, J.A. & Kemshead, J.T. Novel non-isotopic in situ hybridization technique detects small (1 Kb) unique sequences in routinely G-banded human chromosomes: fine mapping of N-myc and beta-NGF genes. Nucleic Acids Res. 15, 4761–4770 (1987).
Acknowledgements
The work presented in this paper was supported in part by a Grant-in-Aid for Scientific Research (B) of the Japan Society for the Promotion of Science to N.K., Life Science Project of 21st Century, Japan, to N.T., and the American Diabetes Association (1-04-ISLET-31) and National Institutes of Health grant 1R21DK60192 to J.W.Y. and H.S.J. We thank Ann Kyle for editorial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Quantification of northern and western blots of islet-specific genes in immortalized and reverted NAK-15 cells. (PDF 109 kb)
Supplementary Fig. 2
Real-time PCR analysis of islet-specific genes in immortalized and reverted NAKT-15 cells. (PDF 74 kb)
Supplementary Fig. 3
Loss of proliferation and lack of tumorigenicity of reverted NAKT-15 cells. (PDF 93 kb)
Supplementary Fig. 4
Insulin secretion and content of NAKT-15 cells reverted at different times during passage in culture. (PDF 64 kb)
Supplementary Table 1
Gene expression of β cell-specific transcription factors and insulin in 253 clones of immortalized human β cells. (PDF 27 kb)
Supplementary Table 2
Insulin secreted in response to glucose and insulin and C-peptide content in reverted NAKT-15 cells in vitro (PDF 67 kb)
Supplementary Table 3
Insulin secreted in response to various secretagogues in reverted NAKT-15 cells in vitro. (PDF 72 kb)
Rights and permissions
About this article
Cite this article
Narushima, M., Kobayashi, N., Okitsu, T. et al. A human β-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol 23, 1274–1282 (2005). https://doi.org/10.1038/nbt1145
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1145
This article is cited by
-
Pancreatic β-cell heterogeneity in adult human islets and stem cell-derived islets
Cellular and Molecular Life Sciences (2023)
-
Modeling different types of diabetes using human pluripotent stem cells
Cellular and Molecular Life Sciences (2021)
-
A prospect of cell immortalization combined with matrix microenvironmental optimization strategy for tissue engineering and regeneration
Cell & Bioscience (2019)
-
Increased expression of microRNAs, miR-20a and miR-326 in PBMCs of patients with type 1 diabetes
Molecular Biology Reports (2018)
-
The state of the art of islet transplantation and cell therapy in type 1 diabetes
Acta Diabetologica (2016)