Abstract
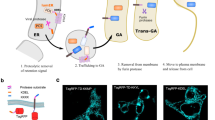
We have previously shown that the intracellular domain of the rabbit prolactin receptor (rbPRL-R), lacking typical signal sequences, was very efficiently secreted into the culture medium when expressed in the baculovirus-insect cell system. We have sought to take advantage of this characteristic for secreting cytoplasmic or nuclear proteins. We have constructed a series of recombinant viruses expressing a foreign gene product fused to the intracellular domain of rbPRL-R. Two passenger genes were used, one encoding a cytoplasmic protein (cyclin B) and the other a nuclear protein (cyclin A). The intracellular domain of rbPRL-R was able to promote the export of these two chimeric proteins with a very high efficiency. This new system should prove useful for secretion of proteins which do not require the post-trans-lational modifications of the classical secretory pathway to be fully active.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Luckow, V.A. 1991. Cloning and expression of heterologous genes in insect cells with baculovirus vectors, p. 97–152. In: Recombinant DNA Technology and Applications. Prokop, A., Bajpan, R. K. and Ho, C. S. (Eds). McGraw-Hill, Inc., N.Y.
Jarvis, D.L. and Summers, M.D. 1992. p. 265–291. In: Recombinant DNA Vaccines: Rationale and Strategies. Isaacson, R. E. (Ed.). Marcel Dekker, N.Y.
Walter, P. and Lingappa, V.R. 1986. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu. Rev. Cell Biol. 2: 499–516.
Tessier, D.C., Thomas, D.Y., Khoury, H.E., Lalberte, F. and Vernet, T. 1991. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin peptkle. Gene 98: 177–183.
Murphy, C.I., McIntire, J.R., Hodgdon, H., Seals, J.R. and Young, E. 1993. Enhanced expression, secretion, and large-scale purification of recombinant HIV-I gpl20 in insect cell using the baculovirus egt and p67 signal peptides. Prot. Exp. Purif. 4: 349–357.
Jarvis, D.L., Summers, M.D., Garcia, A. and Bohlmeyer, D.A. 1993. Influence of different signal peptides and prosequences on expression and secretion of human tissue plasminogen activator in the baculovirus system. J. Biol. Chem. 268: 16754–16762.
Rubartelli, A., Cozzolino, F., Talio, M. and Sitia, R. 1990. A novel secetory pathway for interleukin-lB, a protein lacking a signal sequence. EMBO J. 9: 1503–1510.
Miyamoto, M., Naruo, K.-I., Seko, C., Matsumoto, S., Kondo, T. and Kurokawa, T. 1993. Molecular cloning of a novel cytokine cDNA encoding the ninth member of the fibroblast growth factor family, which has a unique secretion property. Mol. Cel. Biol. 13: 4251–4259.
Holland, I.B., Kenny, B. and Blight, M. 1990. Haemolysin secretion from E. coli. Biochimie 72: 131–141.
Gierse, J.K., Luckow, V.A., Askonas, L.J., Duffin, K.L., Aykent, S., Bild, G.S., Rodi, C.P., Sullivan, M.P., Bourner, M.J., Kimack, N.M. and Krivi, G.G. 1993. High-level expression and purification of human leukotriene A4 hydroxylase from insect cells infected with a baculovirus vector. Prot. Exp. Purif. 4: 358–366.
Nishimura, C., Matsuura, Y., Kokai, Y., Akers, T., Carper, D., Morjana, N., Lyons, C. and Flynn, T.G. 1990. Cloning and expression of human aldolase reductase. J. Biol. Chem. 265: 9788–9792.
Cahoreau, C., Petridou, B., Cerutti, M., Djiane, J. and Devauchelle, G. 1992. Expression of the full-length prolactin receptor and its specific domains in baculovirus infected insect cells. Biochimie 74: 1053–1065.
Cahoreau, C., Gamier, L., Djiane, J., Devauchelle, G. and Cerutti, M. 1994. Evidence for N-glycosylation and ubiquitination of the prolactin receptor expressed in a baculovirus-insect cell system. FEBS Letters 350: 230–234.
Lorca, T., Fesquet, D., Zindy, F., Le Bouffant, F., Cerutti, M., Brechot, C., Devauchelle, G. and Doree, M. 1991. An okadaic acid-sensitive phosphatase negatively controls the cyclin degradation pathway in amphibian eggs. Mol. Cel. Biol. 11: 171–1175.
Kelly, P.A., Ali, S., Rozakis, M., Goujon, L., Nagano, M., Pellegrini, I., Gould, D., Djiane, J., Edery, M., Fimdori, J. and Postel-Vinay, M.C. 1993. The growth hormon/prolactin receptor family, p. 123 In: Recent Progress in Hormon Research, Vol. 48. Academic Press.
Finley, D. and Chau, V. 1991. Ubiquitination. Annu. Rev. Cell Biol. 7: 25–69.
Hershko, A. and Ciechanover, A.A. 1992. The ubiquitin system for protein degradation. Annu. Rev. Biochem. 61: 761–807.
Zhaung, Z. and McCauley, R. 1989. Ubiquitin is involved in the in vitro insertion of monoamine oxydase B into mitochondrial outer membranes. J. Biol. Chem. 264: 14594–14597.
Mackman, N.K., Baker, K., Gray, L., Haigh, R., Nicaud, J.M. and Holland, I.B. 1987. Release of a chimeric protein into the medium from Escherichia coli using the C-terminal secretion signal of haemolysin. EMBO J. 6: 2835–2841.
Koronakis, V., Koronakis, E. and Hughes, C. 1989. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 8: 595–605.
Delepelaire, P. and Wandersman, C. 1991. Characterization, localization and transmembrane organization of the three proteins PrtD, PrtE and PrtF necessary for protease secretion by the Gram-negative bacterium Erwinia chrysanihemi. Mol. Microbiol. 5: 2427–2434.
Wandersman, C. 1992. Secretion across the bacterial outer membrane. Trends Genet. 8: 317–321.
Higgins, C.F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8: 67–113.
Croizier, G., Croizier, L., Quiot, J.M. and Lereclus, D. 1988. Recombination of Autographa californica and Rachiplusia ou nuclear polyhedrosis viruses in Galleria mellonella. J. Gen. Virol. 69: 177–185.
Maniatis, T., Fritsch, E.F. and Sambrook, J. 1982. Molecular Cloning: A Laboratory Manual, p. 545. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
Feigner, P.L. and Ringold, G.M. 1989. Cationic liposome-mediated transfection. Nature 337: 387–388.
Summers, M.D. and Smith, G.E. 1987. A Manual of Methods for Baculovirus Vectors Insect Cell Culture Procedures, p. 1–56. Texas Agric. Exp. St. Bull., no. 1555.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garnier, L., Cahoreau, C., Devauchelle, G. et al. The Intracellular Domain of the Rabbit Prolactin Receptor is Able to Promote the Secretion of a Passenger Protein via an Unusual Secretory Pathway in Lepidopteran Cells. Nat Biotechnol 13, 1101–1104 (1995). https://doi.org/10.1038/nbt1095-1101
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt1095-1101