Abstract
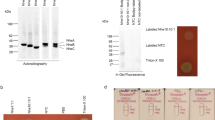
Fragment C is a 50 kD non-toxic polypeptide that is one of the products of cleavage of tetanus toxin by papain. A 90 kD fusion protein containing fragment C when expressed in E. coli was insoluble. In contrast, a polypeptide exactly corresponding to fragment C was expressed in E. coli, in a soluble form, enabling its rapid purification. Recombinant fragment C showed similar biochemical properties and equal effectiveness in immunising mice against tetanus as authentic fragment C derived from C. tetani. The possible use of recombinant fragment C as a tetanus vaccine is discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Stanfield, J.P. and Galazka, A. 1984. Neonatal tetanus in the world today. Bulletin of the World Health Organisation 62:647–669.
Bizzini, B., Turpin, A. and Raynaud, M. 1973. Immunochemistry of tetanus toxin. The nitration of tyrosyl residues in tetanus toxin. Eur. J. Biochem 39:171–181.
Bizzini, B. 1984. Tetanus, p. 37–67. In: Bacterial Vaccines. Germanier, R. (Ed.) Academic Press, NY.
Fairweather, N.F., Lyness, V.A., Pickard, D.J., Allen, G. and Thomson, R.O. 1986. Cloning, nucleotide sequencing and expression of tetanus toxin fragment C in Escherichia coli. J. Bacteriol. 165:21–27.
Fairweather, N.F. and Lyness, V.A. 1986. The complete nucleotide sequence of tetanus toxin. Nucleic Acid Research. 14:7809–7812.
Eisel, U., Jarausch, W., Goretzki, K., Henschen, A., Engels, J., Weller, U., Hudel, M., Habermann, E. and Niemann, H. 1986. Tetanus toxin: primary structure, expression in E. coli and homology with botulinum toxins. EMBO J. 10:2495–2502.
Helting, T.B., Parschat, S. and Engelhardt, H. 1979. Structure of tetanus toxin. Demonstration and separation of a specific enzyme converting intracellular tetanus toxin to the extracellular form. J. Biol Chem. 254:10728–10733.
Matsuda, M. and Yoneda, M. 1974. Dissociation of tetanus neurotoxin into two polypeptide fragments. Biochem. Biophys. Res. Commun. 57:1257–1262.
Helting, T.B. and Zwisler, O. 1977. Structure of tetanus toxin I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J. Biol. Chem. 252:187–193.
Helting, T.B. and Nau, H.H. 1984. Analysis of the immune response to papain digestion products of tetanus toxin. Act. Pathol. Microbiol. Scan. Sect C 92:59–63.
Fairweather, N.F., Lyness, V.A. and Maskell, D.J. 1987. Immunisation of mice against tetanus with fragments of tetanus toxin synthesised in Escherichia coli. Infect. Immun. 55:2541–2545.
Marston, F.A.O. 1986. The purification of eukaryotic polypeptides synthesised in Escherichia coli. Biochem. J. 240:1–12.
Kane, J.F. and Hartley, D.L. 1988. Formation of recombinant protein inclusion bodies in Escherichia coli. Trends In Biotech. 6:95–101.
Makoff, A.J. and Smallwood, A.E. 1988. Heterologous expression in Escherichia coli: effects of alterations in the sequence 5′ to the initiation codon. Biochem. Soc. Trans. 16:48–49.
Bizzini, B., Stoeckel, K. and Schwab, M. 1977. An antigenic polypeptide fragment isolated from tetanus toxin: chemical characterisation, binding to gangliosides and retrograde axonal transport in various neuron systems. J. Neurochem. 28:529–542.
Meselson, M. and Yuan, R. 1968. DNA restriction enzyme from E. coli. Nature 217:1110–1114.
Sanger, F., Nicklen, S. and Coulson, A.R. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U.S.A. 74:5463–5467.
Makoff, A.J., Parry, N. and Dicken, L.P. 1989. Translational fusions with fragments of the trpE gene improve the expression of a poorly expressed heterologous gene in Escherichia coli. J. Gen. Microbiol. 135:11–24
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
Hughes, M., Thompson, R.O., Knight, P. and Stephen, J. 1974. The immunopurification of tetanus toxoid. J. Appl. Bact. 37:603–622.
Sheppard, A.J., Cussell, D. and Hughes, M. 1984. Production and characterisation of monoclonal antibodies to tetanus toxin. Infect. Immun. 43:710–714.
Ballantine, S. and Boxer, D. 1985. Nickel containing hydrogenase isoenzymes from anaerobically grown Escherichia coli. J. Bact. 163:454–459.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Makoff, A., Ballantine, S., Smallwood, A. et al. Expression of Tetanus Toxin Fragment C in E. coli: Its Purification and Potential Use as a Vaccine. Nat Biotechnol 7, 1043–1046 (1989). https://doi.org/10.1038/nbt1089-1043
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt1089-1043