Abstract
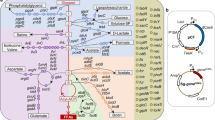
Identification of genes that affect the product accumulation phenotype of recombinant strains is an important problem in industrial strain construction and a central tenet of metabolic engineering. We have used systematic (model-based) and combinatorial (transposon-based) methods to identify gene knockout targets that increase lycopene biosynthesis in strains of Escherichia coli. We show that these two search strategies yield two distinct gene sets, which affect product synthesis either through an increase in precursor availability or through (largely unknown) kinetic or regulatory mechanisms, respectively. Exhaustive exploration of all possible combinations of the above gene sets yielded a unique set of 64 knockout strains spanning the metabolic landscape of systematic and combinatorial gene knockout targets. This included a global maximum strain exhibiting an 8.5-fold product increase over recombinant K12 wild type and a twofold increase over the engineered parental strain. These results were further validated in controlled culture conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stephanopoulos, G., Aristidou, A. & Nielsen, J. Metabolic Engineering: Principles and Methodologies (Academic Press, San Diego, CA, 1998).
Ostergaard, S., Olsson, L., Johnston, M. & Nielsen, J. Increasing galactose consumption by Saccharomyces cerevisiae through metabolic engineering of the GAL gene regulatory network. Nat. Biotechnol. 18, 1283–1286 (2000).
Stafford, D.E. et al. Optimizing bioconversion pathways through systems analysis and metabolic engineering. Proc. Natl. Acad. Sci. USA 99, 1801–1806 (2002).
Koffas, M.A., Jung, G.Y. & Stephanopoulos, G. Engineering metabolism and product formation in Corynebacterium glutamicum by coordinated gene overexpression. Metab. Eng. 5, 32–41 (2003).
Alper, H., Jin, Y.-S., Moxley, J. & Stephanopoulos, G. Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab. Eng. (in the press) 10.1016/j.ymben.2004.12.003 (2005).
Adam, P. et al. Biosynthesis of terpenes: studies on 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase. Proc. Natl. Acad. Sci. USA 99, 12108–12113 (2002).
Matthews, P.D. & Wurtzel, E.T. Metabolic engineering of carotenoid accumulation in Escherichia coli by modulation of the isoprenoid precursor pool with expression of deoxyxylulose phosphate synthase. Appl. Microbiol. Biotechnol. 53, 396–400 (2000).
Misawa, N. & Shimada, H. Metabolic engineering for the production of carotenoids in non-carotenogenic bacteria and yeasts. J. Biotechnol. 59, 169–181 (1997).
Farmer, W.R. & Liao, J.C. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat. Biotechnol. 18, 533–537 (2000).
Smolke, C.D., Martin, V.J.J. & Keasling, J.D. Controlling the metabolic flux through the carotenoid pathway using directed mRNA processing and stabilization. Metab. Eng. 3, 313–321 (2001).
Lee, P.C. & Schmidt-Dannert, C. Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl. Microbiol. Biotechnol. 60, 1–11 (2002).
Kim, S.-W. & Keasling, J.D. Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol. Bioeng. 72, 408–415 (2001).
Jones, K.L., Kim, S.-W. & Keasling, J.D. Low-copy plasmids can perform as well as or better than high-copy plasmids for metabolic engineering of bacteria. Metab. Eng. 2, 328–338 (2000).
Eisen, M., Spellman, P., Brown, P. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863–14868 (1998).
Cunningham, F.X., Jr., Sun, Z., Chamovitz, D., Hirschberg, J. & Gantt, E. Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp strain PCC7942. Plant Cell 6, 1107–1121 (1994).
Maniatis, T., Fritsch, E.F. & Sambrook, J. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1982).
Badarinarayana, V. et al. Selection analyses of insertional mutants using subgenic-resolution arrays. Nat. Biotechnol. 19, 1060–1065 (2001).
Liu, Y.-G. & Whittier, R.F. Thermal asymmetric interlaced pcr: automatable amplification and sequencing of insert end fragments from pi and yac clones for chromosome walking. Genomics 25, 674–681 (1995).
Datsenko, K.A. & Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–6645 (2000).
Miller, J.H. A Short Course in Bacterial Genetics (Cold Springs Harbor Laboratory Press, Cold Springs Harbor, NY, 1992).
Muffler, A., Fischer, D., Altuvia, S., Storz, G. & Hengge-Aronis, R. The response regulator RssB controls stability of the sigma(S) subunit of RNA polymerase in Escherichia coli. EMBO J. 15, 1333–1339 (1996).
Sandmann, G., Woods, W. & Tuveson, R.W. Identification of carotenoids in Erwinia herbicola and in a transformed Escherichia coli strain. FEMS Microbiol. Lett. 59, 77–82 (1990).
Becker-Hapak, M., Troxtel, E., Hoerter, J. & Eisenstark, A. RpoS dependent overexpression of carotenoids from Erwinia herbicola in OXYR-deficient Escherichia coli. Biochem. Biophys. Res. Commun. 239, 305–309 (1997).
Rawlings, N., Tolle, D. & Barrett, A. MEROPS: the peptidase database. Nucleic Acids Res. 32, D160–D164 (2004).
Serres, M.H. et al. A functional update of the Escherichia coli K-12 genome. Genome Biol. 2, published online 20 August 2001 (10.1186/gb-2001-2-9-research0035).
Acknowledgements
We acknowledge financial support of this work by the DuPont-MIT Alliance. In particular, we would like to thank Wonchul Suh for providing the parental E. coli strain. We also thank Joel Moxley for providing thoughtful suggestions and Veronica Godoy for providing the initial phage stock.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Covariance analysis of systematic and combinatorial targets (PDF 113 kb)
Supplementary Table 1
Primer designs for gene knockout constructs (PDF 70 kb)
Rights and permissions
About this article
Cite this article
Alper, H., Miyaoku, K. & Stephanopoulos, G. Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets. Nat Biotechnol 23, 612–616 (2005). https://doi.org/10.1038/nbt1083
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1083
This article is cited by
-
Improvement of lycopene biosynthesis in waaC and waaF mutants of Escherichia coli by integrant expression of crtEBI gene and deletion of aceE and gdhA
Systems Microbiology and Biomanufacturing (2023)
-
Adaptive laboratory evolution of β-caryophyllene producing Saccharomyces cerevisiae
Microbial Cell Factories (2021)
-
Carboxylesterases for the hydrolysis of acetoacetate esters and their applications in terpenoid production using Escherichia coli
Applied Microbiology and Biotechnology (2021)
-
Mastering the control of the Rho transcription factor for biotechnological applications
Applied Microbiology and Biotechnology (2021)
-
CRISPR interference-guided modulation of glucose pathways to boost aconitic acid production in Escherichia coli
Microbial Cell Factories (2020)