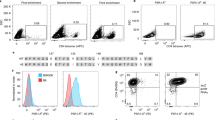
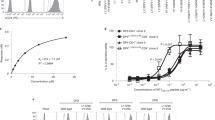
Abstract
In this study we extend tetramerization technology to T-cell receptors (TCRs). We identified TCR αβ pairs in the absence of accessory molecules, ensuring isolation of high-affinity TCRs that maintain stable binding characteristics after tetramerization. Subtle changes in cognate peptide levels bound to the class I molecule were accurately reflected by parallel changes in the mean fluorescence intensity of cells that bound TCR tetramers, allowing us to accurately assess the binding affinity of a panel of peptides to major histocompatibility complex (MHC) class I. Using a TCR tetramer specific for the Mamu-A*01 allele, we identified animals expressing this restricting class I allele from a large cohort of outbred rhesus macaques. TCR tetramers should facilitate analysis of the MHC-peptide interface and, more generally, the design of immunotherapeutics and vaccines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Davis, M.M. et al. Ligand recognition by alpha beta T cell receptors. Annu. Rev. Immunol. 16, 523–544 (1998).
Valitutti, S., Muller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature 375, 148–151 (1995).
Altman, J.D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Kuroda, M.J. et al. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 187, 1373–1381 (1998).
Kuroda, M.J. et al. Human immunodeficiency virus type 1 envelope epitope-specific CD4(+) T lymphocytes in simian/human immunodeficiency virus-infected and vaccinated rhesus monkeys detected using a peptide-major histocompatibility complex class II tetramer. J. Virol. 74, 8751–8756 (2000).
Ogg, G.S. & McMichael, A.J. HLA-peptide tetrameric complexes. Curr. Opin. Immunol. 10, 393–396 (1998).
Holmberg, K., Mariathasan, S., Ohteki, T., Ohashi, P.S. & Gascoigne, N.R. TCR binding kinetics measured with MHC class I tetramers reveal a positive selecting peptide with relatively high affinity for TCR. J. Immunol. 171, 2427–2434 (2003).
Holler, P.D., Chlewicki, L.K. & Kranz, D.M. TCRs with high affinity for foreign pMHC show self-reactivity. Nat. Immunol. 4, 55–62 (2003).
Starr, T.K., Jameson, S.C. & Hogquist, K.A. Positive and negative selection of T cells. Annu. Rev. Immunol. 21, 139–176 (2003).
Holler, P.D. & Kranz, D.M. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 18, 255–264 (2003).
Cho, B.K. et al. Differences in antigen recognition and cytolytic activity of CD8(+) and CD8(−) T cells that express the same antigen-specific receptor. Proc. Natl. Acad. Sci. USA 98, 1723–1727 (2001).
Garcia, K.C. et al. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature 384, 577–581 (1996).
Sykulev, Y. et al. Kinetics and affinity of reactions between an antigen-specific T cell receptor and peptide-MHC complexes. Immunity 1, 15–22 (1994).
Egan, M.A. et al. Use of major histocompatibility complex class I/peptide/beta2M tetramers to quantitate CD8(+) cytotoxic T lymphocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J. Virol. 73, 5466–5472 (1999).
Allen, T.M. et al. CD8+ Lymphocytes from Simian Immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule Mamu-A*01: Implication for vaccine design and testing. J. Virol. 75, 738–749 (2001).
Barouch, D.H. et al. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415, 335–339 (2002).
Adams, E.J. & Parham, P. Species-specific evolution of MHC class I genes in the higher primates. Immunol. Rev. 183, 41–64 (2001).
Boyson, J.E. et al. The MHC class I genes of the rhesus monkey. Different evolutionary histories of MHC class I and II genes in primates. J. Immunol. 156, 4656–4665 (1996).
Lin, A.Y. et al. Expression of T cell antigen receptor heterodimers in a lipid-linked form. Science 249, 677–679 (1990).
Lebowitz, M.S. et al. Soluble, high-affinity dimers of T-cell receptors and class II major histocompatibility complexes: biochemical probes for analysis and modulation of immune responses. Cell. Immunol. 192, 175–184 (1999).
O'Herrin, S.M. et al. Analysis of the expression of peptide-major histocompatibility complexes using high affinity soluble divalent T cell receptors. J. Exp. Med. 186, 1333–1345 (1997).
Plaksin, D., Polakova, K., McPhie, P. & Margulies, D.H. A three-domain T cell receptor is biologically active and specifically stains cell surface MHC/peptide complexes. J. Immunol. 158, 2218–2227 (1997).
Shusta, E.V., Holler, P.D., Kieke, M.C., Kranz, D.M. & Wittrup, K.D. Directed evolution of a stable scaffold for T-cell receptor engineering. Nat. Biotechnol. 18, 754–759 (2000).
Holler, P.D., Lim, A.R., Cho, B.K., Rund, L.A. & Kranz, D.M. CD8(−) T cell transfectants that express a high affinity T cell receptor exhibit enhanced peptide-dependent activation. J. Exp. Med. 194, 1043–1052 (2001).
Kageyama, S., Tsomides, T.J., Sykulev, Y. & Eisen, N.M. Variation in the number of peptide-MHC class I complexes required to activate cytotoxic T cell responses. J. Immunol. 154, 567–576 (1995).
Tsomides, T.J. et al. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J. Exp. Med. 180, 1283–1293 (1994).
Howard, M. & Kaplan, D. Applications of enzymatic amplification staining in immunophenotyping hematopoietic cells. Front. Biosci. 7, c33–c43 (2002).
De Rosa, S.C., Brenchley, J.M. & Roederer, M. Beyond six colors: a new era in flow cytometry. Nat. Med. 9, 112–117 (2003).
Manning, T.C. et al. Alanine scanning mutagenesis of an alphabeta T cell receptor: mapping the energy of antigen recognition. Immunity 8, 413–425 (1998).
Chang, H.C. et al. A general method for facilitating heterodimeric pairing between two proteins: application to expression of alpha and beta T-cell receptor extracellular segments. Proc. Natl. Acad. Sci. USA 91, 11408–11412 (1994).
Acknowledgements
We thank Rudolf P. Bohm, Jr. and Andrew A. Lackner from the Tulane National Primate Research Center (TNPRC) for providing monkey blood samples; and David I. Watkins from the Wisconsin Regional Primate Research Center for the Mamu-A*01 expressing 721.221 cell line used in this study. We thank Carol Lord, Karen Hershberger and William Charini for helpful discussions. This work was supported by National Institutes of Health grants RO1 AI48400 (M.J.K.), AI 48394 (J.E.S.), AI20279 (N.L.L.), AI85343 (N.L.L.), the Dana-Farber Cancer Institute/Beth Israel Deaconess Medical Center/Children's Hospital Center for AIDS Research Grant P30 AI28691 (M.J.K.) and the base grant to the TNPRC RR00168.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Binding of soluble peptide/MCH complexes to the TCR on S2 cells. (PDF 37 kb)
Supplementary Table 1
Peptide binding to Mamu-A*01 (PDF 17 kb)
Rights and permissions
About this article
Cite this article
Subbramanian, R., Moriya, C., Martin, K. et al. Engineered T-cell receptor tetramers bind MHC-peptide complexes with high affinity. Nat Biotechnol 22, 1429–1434 (2004). https://doi.org/10.1038/nbt1024
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1024
This article is cited by
-
Sequence analysis of T-cell repertoires in health and disease
Genome Medicine (2013)
-
Interrogating the repertoire: broadening the scope of peptide–MHC multimer analysis
Nature Reviews Immunology (2011)
-
Stabilizing mutations increase secretion of functional soluble TCR-Ig fusion proteins
BMC Biotechnology (2010)