Abstract
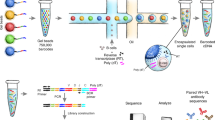
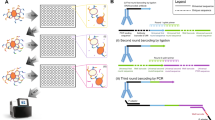
We describe a general approach to rapidly obtain the DNA sequence encoding the variable region of any immunoglobulin chain using the polymerase chain reaction and a mixture of upstream primers corresponding to the leader sequence, and one downstream primer designed from the conserved nucleotide sequence of the constant region. The approach was applied to five different hybridomas producing human monoclonal antibodies and variable regions for both γ and μ heavy chain and κ and λ light chain genes were successfully cloned. cDNA encoding variable regions could be amplified from single hybridoma cells isolated by micromanipulation. This approach will permit analysis of B cell clonal ontogeny, antibody diversity and lymphoma cell progression and heterogeneity. It will also facilitate structural and functional studies of immunoglobulins as well as the rapid construction of chimeric antibodies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Borrebaeck, C.A.K., Danielsson, L., and Möller, S.A. 1988. Human monoclonal antibodies produced by primary in vitro immunization of peripheral blood lymphocytes. Proc. Nad. Acad. Sci. U.S.A. 85:3995–3999.
Borrebaeck, C.A.K. 1988. Human mAbs produced by primary in vitro immunization. Immunol. Today 9:355–359.
Larrick, J.W. 1989. Antibody inhibition of the immunoflammatory cascade. J. Grit. Care In Press.
Larrick, J.W., and Bourla, J.M. 1986. Prospects for the therapeutic use of human monoclonal antibodies. J. Biol. Resp. Mod. 5:379–387.
Morrison, S.L., Johnson, M.J., Hertzenberg, L.A., and Oi, V.T. 1984. Chimeric human antibody molecules: mouse antigen-binding domains with human constant regions domains. Proc. Nad. Acad. Sci. U.S.A. 81:6851–6855.
Riechmann, L., Clark, M., Waldmann, H., and Winter, G. 1988. Reshaping human antibodies for therapy. Nature 332:323–327.
Shawler, D.L., Bartholomew, R.M., Smith, L.M., and Dillman, R.D. 1985. Human immune response to multiple injections of murine monoclonal IgG. J. Immunol. 135:1530–1535.
Jones, P.T., Dear, P.H., Foote, J., Neuberger, M.S., and Winter, G. 1986. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321:522–525.
Verhoeyen, M., Milstein, C., and Winter, G. 1988. Reshaping human antibodies: Grafting an antilysozyme activity. Science 239:1534–1536.
Brown, B.A., Davis, G.L., Saltzgaber-Miiller, J., Simon, P., Ho, M.-K., Shaw, P.S., Stone, B.A., Sands, H., and Moore, G.P. 1987. Tumor-specific genetically engineered murine/human chimeric monoclonal antibody. Cancer Res. 47:3577–3585.
Liu, A.Y., Robinson, R.R, Hellström, K.E., Murrey, E.D. Jr., Chang, C.P., and Hellström, I. 1987. Chimeric mouse-human IgGl antibody that can mediate lysis of cancer cells. Proc. Nad. Acad. Sci. U.S.A. 84:3439–3444.
Morrison, S.L., Canfield, S., Porter, S., Tan, L.K., Tao, M., and Wims, L.A. 1988. Production and characterization of genetically engineered antibody molecules. Clin. Chem. 34:1668–1675.
Saiki, R.K., Scharf, S., Faloona, F., Mullis, K.B., Horn, G.T., Erlich, H.A., and Arnheim, N. 1985. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230:1350–1354.
Scharf, S.J., Horn, G.T., and Erlich, H.A. 1986. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science 233:1076–1079.
Saiki, R.K., Gelfand, D.H., Stoffel, S., Scharff, S.J., Higuchi, R., Horn, G.T., Mullis, K.B., and Erlich, H.A. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–492.
Larrick, J.W., Chiang, Y.L., Sheng-Dong, R., Senyk, G., and Casali, P. 1988. In: In Vitro Immunization in Hybridoma Technology, Progress in Biotechnology 5 (C. A. K. Borrebaeck, Ed.), pp.231—246. Elsevier, Amsterdam.
Rabat, E.A., Wu, T.T., Reid-Miller, M., Perry, H.M., and Gottes-man, K.S. 1987. Sequences of Protein of Immunological Interest, 4th ed. U.S. Dept. Health and Human Services.
Dunning, A.M., Talmud, P., and Humphries, S.E. 1988. Errors in the polymerase chain reaction. Nucl. Acids Res. 16:10393.
Loh, E., Elliott, J.F., Cwirla, S., Lanier, L.L., and Davis, M.M. 1989. Polymerase chain reaction with single-sided specificity: Analysis of T cell receptor δ chain. Science 243:217–220.
von Heijne, G. 1985. Signal sequences: The limits of variation. J. Mol. Biol. 184:99–105.
Larrick, J.W., Danielsson, L., Brenner, C., Abrahamson, M., Fry, K., and Borrebaeck, C.A.K. 1989. Rapid cloning of rearranged immuno-globulin genes from human hybridoma cells using mixed primers and the polymerase chain reaction. Biochem. Biophys. Res. Commun. 160:1250–1256.
Cleary, M.L., Meeker, T.C., Levy, S., Lee, E., Trela, M., Sklar, J., and Levy, R. 1986. Clustering of extensive somatic mutations in the variable region of an immunoglobulin heavy chain gene from a human B cell lymphoma. Cell 44:97–104.
Banapor, B., Rosenthal, K., Rabin, L., Sharma, V., Young, L., Fernan-dez, J., Engleman, E., McGrath, M., Reyes, G., and Lifson, J. 1987. Production, characterization, and epitope mapping of a human monoclonal antibody to the envelope glycoprotein of HIV. J. Immunol. 139:4027–4036.
Rappolee, D.A., Brenner, C.A., Schultz, R., Mark, D., and Werb, Z. 1988. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryo. Science 241:1823–1825.
Messing, J., Groneborn, B., Muller-Hill, B., and Hofschneider, P.H. 1977. Filamentous coliphage M13 as a cloning vehicle: Insertion of a Hindi 11 fragment of the lac regulatory region in M13 replicative form in vitro. Proc. Natl. Acad. Sci. U.S.A. 74:3642–3647.
Vieira, J., and Messing, J. 1982. The pUC plasmids, a M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–264.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Larrick, J., Danielsson, L., Brenner, C. et al. Polymemse Chain Reaction Using Mixed Primers: Cloning of Human Monoclonal Antibody Variable Region Genes from Single Hybridoma Cells. Nat Biotechnol 7, 934–938 (1989). https://doi.org/10.1038/nbt0989-934
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0989-934
This article is cited by
-
The biotechnology and applications of antibody engineering
Molecular Biotechnology (1995)
-
New Techniques for the Production of Therapeutic Recombinant Human Monoclonal Antibodies
Clinical Immunotherapeutics (1995)