Abstract
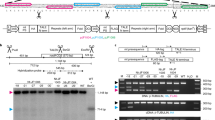
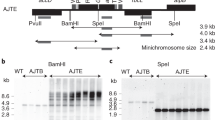
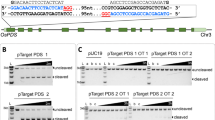
To determine whether targeted DNA insertion into the tobacco plastid genome can be obtained without physical linkage to a selectable marker gene, we carried out biolistic transformation of chloroplasts in tobacco leaf segments with a 1:1 mix of two independently targeted antibiotic resistance genes. Plastid transformants were selected by spectinomycin resistance due to expression of an integrated aadA gene. Integration of the unselected kanamycin resistance (kan) gene into the same plastid genome was established by Southern probing in ∼20% of the spectinomycin-selected clones. Efficient cotransformation will facilitate targeted plastid genome modification without physical linkage to a marker gene.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
McBride, K.E., Svab, Z., Schaaf, D.J., Hogan, P.S., Stalker, D.M. and Maliga, P. 1995. Amplification of a chimeric Bacillus gene in chloroplasts leads to an extraordinary level of an insecticidal protein in tobacco. Bio/Technology 13: 362–365.
Zoubenko, O.V., Allison, L.A., Svab, Z. and Maliga, P. 1994. Efficient targeting of foreign genes into the tobacco plastid genome. Nucl. Acids Res. 22: 3819–3824.
Staub, J. and Maliga, P. 1994. Translation of psbA mRNA is regulated by light via the 5′-untranslated region in tobacco plastids. Plant. J. 6: 547–553.
McBride, K.E., Schaaf, D.J., Daley, M. and Stalker, D.M. 1994. Controlled expression of plastid transgenes in plants based on a nuclear DNA-encoded and plastid-targeted T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 91: 7301–7305.
Sutton, C.A., Zoubenko, O.V., Hanson, M.R. and Maliga, P. 1995. A plant mitochondrial sequence transcribed in transgenic tobacco chloroplasts is not edited. Mol. Cell. Biol. 15: 1377–1381.
Maliga, P. 1993. Towards plastid transformation in flowering plants. Trends in Biotech. 11: 101–107.
Maliga, P., Carrer, H., Kanevski, I., Staub, J. and Svab, Z. 1993. Plastid engineering in land plants: a conservative genome is open to change. Phil. Trans. R. Soc. Lond. B 342: 203–208.
Bock, R., Kossel, H. and Maliga, P. 1994. Introduction of a heterologous editing site into the tobacco plastid genome: lack of RNA editing leads to a mutant phenotype. EMBO J. 13: 4623–4628.
Bock, R. and Maliga, P. 1995. In vivo testing of a tobacco plastid DNA segment for guide RNA function in psbL editing. Molec. Gen. Genet. 247: 439–443.
Newman, S.M., Gillham, N.W., Harris, E.H., Johnson, A.M. and Boynton, J.E. 1991. Targeted disruption of chloroplast genes in Chlamydomonas reinhardtii. Mol. Gen. Genet. 230: 65–74.
Kindle, K.L., Richards, K.L. and Stern, D.B. 1991. Engineering the chloroplast genome: techniques and capabilities for chloroplast transformation in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 88: 1721–1725
Roffey, R.A., Golbeck, J.H., Hille, C.R. and Syre, R.T. 1991. Photosynthetic electron transport in genetically altered photosystem II reaction centers of chloroplasts. Proc. Natl. Acad. Sci. USA 88: 9122–9126.
Boynton, J.E. and Gillham, N.K. 1993. Chloroplast transformation in Chlamydomonas. Methods in Enzymol. 217: 510–536.
Cannon, G., Heinhorst, S., Siedlecki, J. and Weissbach, A. 1985. Chloroplast DNA synthesis in light and dark grown cultured Nicotiana tabacum cells as determined by molecular hybridization. Plant Cell Reports 4: 41–45.
Yasuda, T., Kuroiwa, T. and Nagata, T. 1988. Preferential synthesis of plastid DNA and increased replication of plastids in cultured tobacco cells following medium renewal. Planta 174: 235–241.
Thomas, M.R. and Rose, R.J. 1983. Plastid number and plastid structural changes associated with tobacco mesophyll protoplast culture and plant regeneration. Planta 158: 329–338.
Svab, Z. and Maliga, P. 1993. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc. Natl. Acad. Sci. USA 90: 913–917.
Carrer, H., Hockenberry, T.N., Svab, Z. and Maliga, P. 1993. Kanamycin resistance as a selectable marker for plastid transformation in tobacco. Molec. Gen. Genet. 241: 49–56.
Boynton, J.E., Gillham, N.W., Harris, E.H., Hosler, J.P., Johnson, A.M., Jones, A.R., Randolph-Anderson, B.L., Robertson, D., Klein, T.M., Shark, K.B. and Sanford, J.C. 1988. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 240: 1534–1537.
Kuroiwa, T. 1991. The replication, differentiation, and inheritance of plastids with emphasis in the concept of organelle nuclei. Int. Rev. Cytol. 128: 1–62.
Liu, J.-W. and Rose, R.J. 1992. The spinach chloroplast chromosome is bound to the thylacoid membrane in the region of the inverted repeat. Biochem. Biophys. Res. Comm. 148: 993–1000.
Sato, N., Albrieux, C., Joyard, J., Douce, R. and Kuroiwa, T. 1993. Detection and characterization of a plastid envelope DNA-binding protein which may anchor plastid nucleoids. EMBO J. 12: 555–561.
Vieira, J. and Messing, J. 1987. Production of single-stranded plasmid DNA. Methods in Enzymol. 153: 3–11.
Shinozaki, K., Ohme, M., Tanaka, M., Wakasugi, T., et al. 1986. The complete nucleotide sequence of the tobacco chloroplast genome: Its gene organization and expression. EMBO J. 5: 2043–2049.
Murashige, T. and Skoog, F. 1962. A revised medium for the growth and bioassay with tobacco tissue culture. Physiol. Plant. 15: 473–497.
Carrer, H., Staub, J.M. and Maliga, P. 1991. Gentamycin resistance in Nicotiana conferred by AAC(3)-I, a narrow substrate specificity acetyltransferase. Plant Mol Biol. 17: 301–303.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carrer, H., Maliga, P. Targeted Insertion of Foreign Genes into the Tobacco Plastid Genome without Physical Linkage to the Selectable Marker Gene. Nat Biotechnol 13, 791–794 (1995). https://doi.org/10.1038/nbt0895-791
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0895-791
This article is cited by
-
Strategies for metabolic pathway engineering with multiple transgenes
Plant Molecular Biology (2013)
-
Next generation synthetic vectors for transformation of the plastid genome of higher plants
Plant Molecular Biology (2009)
-
Stable chloroplast transformation in cabbage (Brassica oleracea L. var. capitata L.) by particle bombardment
Plant Cell Reports (2007)
-
Development of Novel Types of Plastid Transformation Vectors and Evaluation of Factors Controlling Expression
Transgenic Research (2005)