Abstract
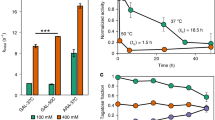
We have engineered recombinant glucose isomerase (GI) from Actinoplanes missouriensis by site-directed mutagenesis to enhance its thermal stability in both the soluble and immobilized forms. Substitution of arginine for lysine at position 253, which lies at the dimer/dimer interface of the GI tetramer, produced the largest stabilization under model industrial conditions. We discuss our results in terms of a model in which chemical glycation of lysines by sugars in the industrial corn syrup substrate represents a major pathway of destabilization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Chen, W.-P. 1980. Glucose isomerase (a review). Process Biochem. June/July: 30–35.
Chen, W.-P. 1980. Glucose isomerase (a review). Process Biochem. August/Sept: 36–41.
Jensen, V.J. and Rugh, S. 1987. Industrial-scale production and application of immobilized glucose isomerase. Methods Enzymol. 136: 356–370.
Farber, G.K. 1987. The 3 Å crystal structure of xylose isomerase from Streptomyces olivochromogenes. Protein Eng. 1: 459–466.
Henrick, K., Blow, D.B., Carrell, H.L. and Glusker, J.P. 1987. Comparison of the backbone structures of glucose isomerase from Streptomyces and Arthrobacter. Protein Eng. 1: 467–469.
Rey, F., Jenkins, J., Janin, J., Lasters, I., Alard, P., Claessens, M., Matthyssens, G. and Wodak, S. 1988. Structural analysis of the 2.8 Å model of xylose isomerase from Actinoplanes missouriensis. Proteins 4: 165–172.
Miller, S., Lesk, A.M., Janin, J. and Chotia, C. 1987. The accessible surface area and stability of oligomeric proteins. Nature 27: 834–836.
Bunn, H.F., Haney, D.N., Gabbay, K.N. and Gallop, P.M. 1975. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c Biochem. Biophys. Res. Comm. 67: 103–109.
Alber, T. 1989. Mutational effects on protein stability. Ann. Rev. Biochem. 58: 765–798.
Matthews, B.W., Nicholson, H. and Becktel, W.J. 1987. Enhanced protein stability from site-directed mutations that decrease the entropy of unfolding. Proc. Natl. Acad. Sci. U.S.A. 84: 6663–6667.
Hecht, H.M., Sturtevant, J.M. and Sauer, R.T. 1984. Stabilization of lambda represser against thermal denaturation by site-directed Gly to Ala changes in α-helix three. Proc. Natl. Acad. Sci. U.S.A. 81: 5685–5689.
Alber, T., Sun, D.-P., Nye, J.A., Muchmore, D.C. and Matthews, B.W. 1987. Temperature-sensitive mutations of bacteriophage T4 lysozyme occur at sites of low solvent accessibility in the folded protein. Biochemistry 26: 3754–3758.
Wigley, D.B., Clarke, A.R., Dunn, C.R., Barstow, D.A., Atkinson, T., Chia, W.N., Muirhead, H. and Holbrook, J.J. 1987. The engineering of a more thermally stable lactate dehydrogenase by reduction of the area of water-accessible hydrophobic surface. Biochim. Biophyc. Acta 916: 145–148.
Howard Flanders, P., Boyce, R.P. and Theriot, L. 1966. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagenic products from DNA. Genetics 53: 1119–1136.
Stanssens, P., Opsomer, C., Beyart, M., van Vliet, A. and Lauwereys, M. Production, purification and characterization of recombinant Actinoplanes missouriensis D-xylose isomerase. Submitted.
Stanssens, P., Opsomer, C., McKeown, Y.M., Kramer, W., Zabeau, M. and Fritz, H.J. 1989. Efficient oligonucleotide directed construction of mutations in expression vectors by the gapped duplex DNA method using alternating selectable markers. Nuc. Acids Res. 17: 4441–4454.
Colson, C., Clocer, S.W., Sijmonds, N. and Stacy, K. 1965. The location of the genes for host controlled modification and restriction in Escherichia coli K-12. Genetics 52: 1043–1050.
Mrabet, N.T., van den Broeck, A., van den Brande, I., Stanssens, P. et al. 1991. Argine residues as stabilizing elements in proteins. Biochemistry. In press.
Furth, A. 1988. Sweet perils for proteins. New Scientist 117: 58–62.
Carrell, H.L., Glusker, J.P., Burger, V., Tritsch, D. and Bieleman, J.-F. 1989. X-ray analysis of D-xylose isomerase at 1.9 Å: Native enzyme in complex with substrate and with a mechanism designed inactivator. Proc. Natl. Acad. Sci. U.S.A. 86: 4440–4444.
Levitt, M. 1978. Conformational preferences of amino acids in globular proteins. Biochemistry 17: 4277–4285.
Powell, L.W. 1984. Developments in immobilized-enzyme technology. Biotechnology and Genetic Engineering Rev. 2: 409–438.
Klibanov, A.M. 1982. Immobilized enzymes and cells as practical catalysts. Science 219: 722–727.
Van Tilburg, R. and Roels, J.A. 1982. An improved productivity and stability test for immobilized enzymes with reference to glucose isomerase. Die Stärke 34: 134–140.
Furth, A.J. 1988. Methods for assaying non-enzymatic glycosylation. Anal. Biochem. 175: 347–360.
Volkin, D.B. and Klibanov, A.M. 1989. Mechanism of thermoinactivation of immobilized glucose isomerase. Biotechnol. Bioeng. 33: 1104–1111.
Amore, A. and Hollenberg, C.P. 1989. Xylose isomerase from Actinoplanes missouriensis: primary structure of the gene and the protein. Nuc. Acids Res. 17: 7515.
Farber, G.K., Glasfeld, A., Tiraby, G., Ringe, D. and Petsko, G.A. 1989. Crystallographic studies of the mechanism of xylose isomerase. Biochemistry 28: 7289–7297.
Callens, M., Kersters-Hilderson, H., Van Opstal, O. and DeBruyne, C.K. 1986. Catalytic properties of D-xylose isomerase from Streptomyces violaceoruber. Enzyme Microb. Technol. 8: 696–700.
Roels, J.A. and van Tilburg, R. 1979. Temperature dependence of stability and activity of an immobilized glucose isomerase in a packed bed. Die Stärke 31: 17–24.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Quax, W., Mrabet, N., Luiten, R. et al. Enhancing the Thermostability of Glucose Isomerase by Protein Engineering. Nat Biotechnol 9, 738–742 (1991). https://doi.org/10.1038/nbt0891-738
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0891-738
This article is cited by
-
Thermal Stabilization of Xylose Isomerase from Thermoanaerobacterium thermosulfurigenes
Nature Biotechnology (1993)