Abstract
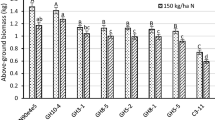
Dinitroaniline herbicides are used for the selective control of weeds in arable crops. Dinitroaniline herbicide resistance in the invasive weed goosegrass was previously shown to stem from a spontaneous mutation in an α-tubulin gene. We transformed and regenerated tobacco plants with an α/β-tubulin double gene construct containing the mutant α-tubulin gene and showed that expression of this construct confers a stably inherited dinitroaniline-resistant phenotype in tobacco. In all transformed lines, the transgene α- and β-tubulins increased the cytoplasmic pool of tubulin approximately 1.5-fold while repressing endogenous α- and β-tubulin synthesis by up to 45% in some tissues. Transgene α- and β-tubulin were overexpressed in every plant tissue analyzed and comprised approximately 66% of the total tubulin in these tissues. Immunolocalization studies revealed that transgene α- and β-tubulins were incorporated into all four microtubule arrays, indicating that they are functional. The majority of the α/β- tubulin pools are encoded by the transgenes, which implies that the mutant α-tubulin and the β-tubulin can perform the majority, if not all, of the roles of microtubules in both juvenile and adult tobacco plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sumida, S. & Ueda, M. Effect of O-ethyl O-(3-methyl-6-nitrophenyl) N-sec-butyl-phosphoroamidate (S-2846), an experimental herbicide, on mitosis in Allium cepa. Plant Cell Pysiol. 17, 1351–1354 (1976).
Appleby, A.P. & Valverde, B.E. Behaviour of dinitroaniline herbicides in plants. Weed Technol. 3, 198– 206 (1989).
Anthony, R.G. & Hussey, P.J. Dinitroaniline herbicide resistance and the microtubule cytoskeleton. Trends Plant Sci. 4, 112–116 (1999).
Morejohn, L.C., Bureau, T.E., Mole-Bajer, J., Bajer, A.S. & Fosket, D.E. Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro . Planta 172, 252–264 (1987).
Mudge, L.C., Gossett, B.J. & Murphy, T.R. Resistance of goosegrass (Eleusine indica) to dinitroaniline herbicides. Weed Sci. 32, 591–594 (1984).
Vaughn, K.C., Marks, M.D. & Weeks, D.P. A dinitroaniline resistant mutant of Eleusine indica exhibits cross-resistance and supersensitivity to antimicrotubule herbicides and drugs. Plant Physiol. 83, 956– 964 (1987).
Waldin, T.R., Ellis, J.R. & Hussey, P.J. Tubulin-isotype analysis of two grass species-resistant to dinitroaniline herbicides. Planta 188, 258–264 (1992).
Cronin, K.E., Hussey, P.J., Ray, J.A. & Waldin, T.R. Herbicide resistant plants. World Intellectual Property Organization No. WO 93/ 24637 (1993).
Anthony, R.G., Waldin, T.R., Ray, J.A., Bright, S.W.J. & Hussey, P.J. Herbicide resistance caused by spontaneous mutation of the cytoskeletal protein tubulin. Nature 393, 260–263 (1998).
Yamamoto, E., Zeng, L. & Baird, W.V. α-tubulin missense mutations correlate with antimicrotubule drug resistance in Eleusine indica. Plant Cell 10, 297–308 (1998).
Anthony, R.G. & Hussey, P.J. Suppression of endogenous α - and β-tubulin synthesis in transgenic maize calli overexpressing α - and β-tubulins. Plant J. 16, 297– 304 (1998).
Raff, E.C. in Microtubules (eds Hyams, J. & Lloyd, C.) 85– 109 (Wiley-Liss, New York 1994).
Hussey P.J. et al. The β-tubulin gene family in Zea mays: two differentially expressed tubulin genes. Plant Mol. Biol. 15, 957–972 (1990).
Ellis, J.R., Taylor, R. & Hussey, P.J. Molecular modelling indicates that two chemically distinct classes of anti-mitotic herbicide bind to the same receptor site(s). Plant Physiol. 105, 15– 18 (1994).
Cleveland, D.W. & Theodorakis, N.G. in Microtubules (eds Hyams, J. & Lloyd, C.) 47–58 (Wiley-Liss, New York 1994).
Gonzalez-Garay, M.L. & Cabral, F. α-tubulin limits its own synthesis: evidence for a mechanism involving translational repression. J. Cell Biol. 135, 1525– 1534 (1996).
Ausubel, F.M. et al. Current protocols in molecular biology. (Greene Publishing Associates, New York; 1992).
Horsch, R.S. et al. A simple and general method for transferring genes into plants. Science 227, 1229–1231 (1985).
Doyle, J.J. & Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 12, 13–15 (1990).
Hussey, P.J. & Gull, K. Multiple isotypes of α and β-tubulin in the plant Phaseolus vulgaris. FEBS Lett. 181, 131–118 (1985).
Smertenko, A., Blume, Y., Viklicky, V., Opatrny Z. & Draber, P. Post-translational modifications and multiple tubulin isoforms in Nicotiana tabacum L cells. Planta 201, 349–358 ( 1997).
Field, J. et al. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition. Meth. Mol. Cell. Biol. 8, 2159–2165 (1988).
Acknowledgements
This work was funded by the Biotechnological and Biological Sciences Research Council. We would like to thank J. Payne (Zeneca Agrochemicals) for assistance with the tobacco transformation technique.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anthony, R., Reichelt, S. & Hussey, P. Dinitroaniline herbicide-resistant transgenic tobacco plants generated by co-overexpression of a mutant α-tubulin and a β-tubulin. Nat Biotechnol 17, 712–716 (1999). https://doi.org/10.1038/10931
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/10931
This article is cited by
-
Overexpression of α-Tubulin Gene of Sugarcane (Saccharum spp. hybrids), SoTUA, Enhances Tobacco Tolerance to Cold Stress
Sugar Tech (2022)
-
Characterization and putative post-translational regulation of α- and β-tubulin gene families in Salix arbutifolia
Scientific Reports (2016)
-
Oryzalin bodies: in addition to its anti-microtubule properties, the dinitroaniline herbicide oryzalin causes nodulation of the endoplasmic reticulum
Protoplasma (2009)
-
Multiherbicide tolerance conferred by AtPgp1 and apyrase overexpression in Arabidopsis thaliana
Nature Biotechnology (2003)