Abstract
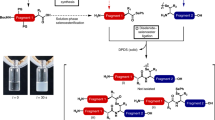
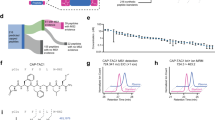
We have made an active analogue of salmon calcitonin I, [homoserine 31]–salmon calcitonin I, by recombinant DNA and chemical techniques. First a calcitonin precursor, the [homoserine31]–salmon calcitonin I–(1–31) lactone, was obtained from a multi–copy fusion protein upon cyanogen bromide cleavage. The protein was expressed from a multiple–copy salmon calcitonin I gene fused to lacZ. The C–terminal homoserine was reacted with prolinamide to get the 32 amino acid peptide, [homoserine31]–salmon calcitonin I. This analogue was equipotent to the naturally–occurring salmon calcitonin I in lowering plasma calcium levels in a rat bioassay. This method thus offers an opportunity to make C–terminal amides through intermediates made by recombinant DNA methods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tatemoto, K., Mutt, V. 1978 Chemical determination of polypeptide hormones. Proc. Natl. Acad. Sci. USA 75:4115–4119.
Niall, H.D., Keutmann, H.T., Copp, D.H., Potts, J.T., Jr., 1969 Amino acid sequence of salmon ultimobranchial calcitonin. Proc. Natl. Acad. Sci. 64:771–778.
Kempe, T., Kent, S.B.H., Chow, F., Peterson, S.M., Sundquist, W.I., L'ltalien, J.J., Harbrecht, D., Plunkett, D., DeLorbe, W.J. 1985. Multiple-copy genes: production and modification of monomeric peptides from large multimeric fusion proteins. Gene 39:239–245.
Kempe, T., Chow, F., Peterson, S.M., Baker, P., Hays, W., Opperman, G., L'Italien, J.J., Long, G., Paulson, B. 1986. Production and characterization of growth hormone releasing factor analogs through recombinant DNA and chemical techniques. Bio/Technoloey 4:565–568.
Chow, F., Kempe, T., Palm, G. 1981. Synthesis of oligodeoxyribonucleotides on silica gel support. Nucleic Acids Research 9:2807–2817.
Beaucage, S.L., Caruthers, M.H. 1981. Deoxynucleoside phosphoramidites—a new class of key intermediates for deoxypolynucleotide synthesis. Tetrahedron Letters 22:1859–1862.
Grosjean, H., Fiers, W. 1982. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene 18:199–209.
Watson, R.J., Weis, J.H., Salstrom, J.S., Enquist, L.W. 1985. Expression of Herpes Simplex virus type I and type II glycoprotein D genes using the “Escherichia Coli Lac” promoter, p. 327—352. In: Recombinant DNA Research and Viruses. T. Becker (ed.). Martinus Nijhoff, The Hague.
Halling, S.M., Smith, S. 1985. Expression in Escherchia coli of multiple products from a chimaeric gene fusion: evidence for the presence of procaryotic translational control regions within eucaryotic genes. Bio/Technology 3:715–720.
Gross, E., Witkop, B. 1961. Selective cleavage of the methionyl peptide bonds in ribonuclease with cyanogen bromide. J. Am. Chem. Soc. 83:1510–1511.
Dyckes, D.F., Kina, H., Sheppard, R.C. 1977. Studies on the partial synthesis of protein analogs by direct coupling to terminal homoserine lactone derivatives. Int. J. Peptide Protein Res. 9:340–348.
Doyen, N., Lapresle, C. 1979. Partial non-cleavage by cyanogen bromide of a methionine-cystine bond from human serum albumin and bovine α-lactalbumin. Biochem. J. 177:251–254.
Bidlingmyer, B.A., Cohen, S.A., Tarvin, T.L. 1984. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. 336:93–104.
Hewick, R.M., Hunkapiller, M.W., Hood, L.E., Dryer, W.J. 1981. A gas liquid solid phase peptide and protein sequenator. J. Biol. Chem. 256:7990–7997.
Tarr, G.E. 1981. Separation of amino acid phenylthiohydantoins by isocratic high performance liquid chromatography. Anal. Biochem. 111:27–32.
Rittel, W., Maier, R., Brugger, M., Kambeer, B., Riniker, B., Sieber, P. 1976. Structure activity relationship of human CT. III. Biological activity of synthetic analogues with shortened or terminally modified peptide chains. Experientia 32:246–249.
Austin, L.A., Heath, H. III. 1981. Medical Progress. Calcitonin: Physiology and pathophysiology. N. Engl. J. Med. 304:269–278.
Heath, H., Sizemore, G.W., 1982. Radioimmunoassay for calcitonin. Clin. Chem. 28:1219–1226.
Heath, H. III, DiBella, F.P., 1978. Reduced-volume radioimmunoassays for parathyrin and calcitonin in serum, for use in pediatric and small animal studies. Clin. Chem. 24:1833–1835.
Sturtridge, W.C., Kumar, M.A., 1968. An improved bioassay for calcitonin. J. Endocr. 42:501–503.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kempe, T., Chow, F., Fass, D. et al. [Homoserine31]-Salmon Calcitonin I: Fully Active Analogue of Calcitonin Synthesized by Recombinant DNA Techniques. Nat Biotechnol 6, 190–192 (1988). https://doi.org/10.1038/nbt0288-190
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0288-190