Abstract
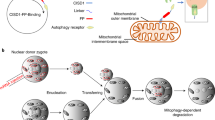
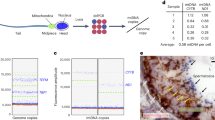
Mitochondria are essential cytoplasmic organelles that generate energy (ATP) by oxidative phosphorylation and mediate key cellular processes such as apoptosis. They are maternally inherited and in humans contain a 16,569-base-pair circular genome (mtDNA) encoding 37 genes required for oxidative phosphorylation. Mutations in mtDNA cause a range of pathologies, commonly affecting energy-demanding tissues such as muscle and brain. Because mitochondrial diseases are incurable, attention has focused on limiting the inheritance of pathogenic mtDNA by mitochondrial replacement therapy (MRT). MRT aims to avoid pathogenic mtDNA transmission between generations by maternal spindle transfer, pronuclear transfer or polar body transfer: all involve the transfer of nuclear DNA from an egg or zygote containing defective mitochondria to a corresponding egg or zygote with normal mitochondria. Here we review recent developments in animal and human models of MRT and the underlying biology. These have led to potential clinical applications; we identify challenges to their technical refinement.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
14 December 2017
In the version of this article initially published, in Table 2, first column, “m.13095T > C” should have been “m.130bT > C,” where “b” refers to the footnote “Characters hidden to respect confidentiality,” as with the other three from the Newcastle Group. In addition, the footnote “a” for Table 2 should have read “http://hfeaarchive.uksouth.cloudapp.azure.com/www.hfea.gov.uk/docs/Fourth_scientific_review_mitochondria_2016.pdf” rather than “Personal communication.” The following acknowledgment was omitted: “The authors thank Rob Taylor, Charlotte Alston, Emma Watson, Sam Byerley, Jane Stewart and Robert McFarland (Wellcome Centre for Mitochondrial Research Newcastle University and Newcastle upon Tyne Hospitals NHS Foundation Trust) for unpublished data included in Table 2.” The errors have been corrected in the HTML and PDF versions of the article.
References
Chinnery, P.F. & Hudson, G. Mitochondrial genetics. Br. Med. Bull. 106, 135–159 (2013).
Cree, L.M. et al. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat. Genet. 40, 249–254 (2008).
Otten, A.B. & Smeets, H.J. Evolutionary defined role of the mitochondrial DNA in fertility, disease and ageing. Hum. Reprod. Update 21, 671–689 (2015).
Cao, L. et al. New evidence confirms that the mitochondrial bottleneck is generated without reduction of mitochondrial DNA content in early primordial germ cells of mice. PLoS Genet. 5, e1000756 (2009).
Anderson, S. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 (1981).
Wai, T., Teoli, D. & Shoubridge, E.A. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat. Genet. 40, 1484–1488 (2008).
Song, W.H., Ballard, J.W., Yi, Y.J. & Sutovsky, P. Regulation of mitochondrial genome inheritance by autophagy and ubiquitin-proteasome system: implications for health, fitness, and fertility. BioMed Res. Int. 2014, 981867 (2014).
Kaneda, H. et al. Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proc. Natl. Acad. Sci. USA 92, 4542–4546 (1995).
Shitara, H. et al. Selective and continuous elimination of mitochondria microinjected into mouse eggs from spermatids, but not from liver cells, occurs throughout embryogenesis. Genetics 156, 1277–1284 (2000).
Song, W.H., Yi, Y.J., Sutovsky, M., Meyers, S. & Sutovsky, P. Autophagy and ubiquitin-proteasome system contribute to sperm mitophagy after mammalian fertilization. Proc. Natl. Acad. Sci. USA 113, E5261–E5270 (2016).
Luo, S.M. et al. Unique insights into maternal mitochondrial inheritance in mice. Proc. Natl. Acad. Sci. USA 110, 13038–13043 (2013).
Chinnery, P.F., Andrews, R.M., Turnbull, D.M. & Howell, N.N. Leber hereditary optic neuropathy: does heteroplasmy influence the inheritance and expression of the G11778A mitochondrial DNA mutation? Am. J. Med. Genet. 98, 235–243 (2001).
Caporali, L. et al. Incomplete penetrance in mitochondrial optic neuropathies. Mitochondrion 36, 130–137 (2017).
Poulton, J., Finsterer, J. & Yu Wai Man, P. Genetic counselling for maternally inherited mitochondrial disorders. Mol. Diagn. Ther. 21, 419–429 (2017).
Steffann, J. et al. Analysis of mtDNA variant segregation during early human embryonic development: a tool for successful NARP preimplantation diagnosis. J. Med. Genet. 43, 244–247 (2006).
Mitalipov, S., Amato, P., Parry, S. & Falk, M.J. Limitations of preimplantation genetic diagnosis for mitochondrial DNA diseases. Cell Reports 7, 935–937 (2014).
Mann, J.R. & Lovell-Badge, R.H. Inviability of parthenogenones is determined by pronuclei, not egg cytoplasm. Nature 310, 66–67 (1984).
Kimura, Y. & Yanagimachi, R. Development of normal mice from oocytes injected with secondary spermatocyte nuclei. Biol. Reprod. 53, 855–862 (1995).
Wang, M.K., Chen, D.Y., Liu, J.L., Li, G.P. & Sun, Q.Y. In vitro fertilisation of mouse oocytes reconstructed by transfer of metaphase II chromosomes results in live births. Zygote 9, 9–14 (2001).
Wakayama, T. & Yanagimachi, R. The first polar body can be used for the production of normal offspring in mice. Biol. Reprod. 59, 100–104 (1998).
Wakayama, T., Hayashi, Y. & Ogura, A. Participation of the female pronucleus derived from the second polar body in full embryonic development of mice. J. Reprod. Fertil. 110, 263–266 (1997).
Wakayama, T., Perry, A.C., Zuccotti, M., Johnson, K.R. & Yanagimachi, R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 394, 369–374 (1998).
Inoue, K. et al. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat. Genet. 26, 176–181 (2000).
Nakada, K., Inoue, K. & Hayashi, J.I. Mito-mice: animal models for mitochondrial DNA-based diseases. Semin. Cell Dev. Biol. 12, 459–465 (2001).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Trifunovic, A. et al. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc. Natl. Acad. Sci. USA 102, 17993–17998 (2005).
Kauppila, T.E.S., Kauppila, J.H.K. & Larsson, N.G. Mammalian mitochondria and aging: an update. Cell Metab. 25, 57–71 (2017).
Hayashi, J.I., Hashizume, O., Ishikawa, K. & Shimizu, A. Mutations in mitochondrial DNA regulate mitochondrial diseases and metastasis but do not regulate aging. Curr. Opin. Genet. Dev. 38, 63–67 (2016).
Latorre-Pellicer, A. et al. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 535, 561–565 (2016).
Saben, J.L. et al. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 16, 1–8 (2016).
Boudoures, A.L. et al. Obesity-exposed oocytes accumulate and transmit damaged mitochondria due to an inability to activate mitophagy. Dev. Biol. 426, 126–138 (2017).
Ma, H. et al. Incompatibility between nuclear and mitochondrial genomes contributes to an interspecies reproductive barrier. Cell Metab. 24, 283–294 (2016).
Lee, H.S. et al. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Rep. 1, 506–515 (2012).
Wang, T. et al. Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases. Cell 157, 1591–1604 (2014).
Tachibana, M. et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature 493, 627–631 (2013).
Paull, D. et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature 493, 632–637 (2013).
Craven, L. et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 465, 82–85 (2010).
Kang, E. et al. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature 540, 270–275 (2016).
Yamada, M. et al. Genetic drift can compromise mitochondrial replacement by nuclear transfer in human oocytes. Cell Stem Cell 18, 749–754 (2016).
Hyslop, L.A. et al. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature 534, 383–386 (2016).
Ma, H. et al. Functional Human Oocytes Generated by Transfer of Polar Body Genomes. Cell Stem Cell 20, 112–119 (2017).
Yamada, M. et al. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature 510, 533–536 (2014).
Palacios-González, C. & Medina-Arellano, M.J. Mitochondrial replacement techniques and Mexico's rule of law: on the legality of the first maternal spindle transfer case. J. Law Biosci. 4, 50–69 (2017).
Ishii, T. Potential impact of human mitochondrial replacement on global policy regarding germline gene modification. Reprod. Biomed. Online 29, 150–155 (2014).
Zhang, J. et al. Pregnancy derived from human zygote pronuclear transfer in a patient who had arrested embryos after IVF. Reprod. Biomed. Online 33, 529–533 (2016).
Zhang, J. et al. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. Biomed. Online 34, 361–368 (2017).
Alikani, M., Fauser, B.C.J., García-Valesco, J.A., Simpson, J.L. & Johnson, M.H. First birth following spindle transfer for mitochondrial replacement therapy: hope and trepidation. Reprod. Biomed. Online 34, 333–336 (2017).
Quirós, P.M., Mottis, A. & Auwerx, J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 17, 213–226 (2016).
Akman, G. et al. Pathological ribonuclease H1 causes R-loop depletion and aberrant DNA segregation in mitochondria. Proc. Natl. Acad. Sci. USA 113, E4276–E4285 (2016).
Wolf, D.P., Hayama, T. & Mitalipov, S. Mitochondrial genome inheritance and replacement in the human germline. EMBO J. 36, 2659 (2017).
Reddy, P. et al. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell 161, 459–469 (2015).
Reinhardt, K., Dowling, D.K. & Morrow, E.H. Medicine. Mitochondrial replacement, evolution, and the clinic. Science 341, 1345–1346 (2013).
Chinnery, P.F. et al. The challenges of mitochondrial replacement. PLoS Genet. 10, e1004315 (2014).
Li, M., Schröder, R., Ni, S., Madea, B. & Stoneking, M. Extensive tissue-related and allele-related mtDNA heteroplasmy suggests positive selection for somatic mutations. Proc. Natl. Acad. Sci. USA 112, 2491–2496 (2015).
Eyre-Walker, A. Mitochondrial replacement therapy: are mito-nuclear interactions likely to be a problem? Genetics 205, 1365–1372 (2017).
Rishishwar, L. & Jordan, I.K. Implications of human evolution and admixture for mitochondrial replacement therapy. BMC Genomics 18, 140 (2017).
Ledford, H. AstraZeneca launches project to sequence 2 million genomes. Nature 532, 427 (2016).
Sallevelt, S.C. et al. De novo mtDNA point mutations are common and have a low recurrence risk. J. Med. Genet. 54, 73–83 (2017).
Dalton, C.M. & Carroll, J. Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte. J. Cell Sci. 126, 2955–2964 (2013).
Steuerwald, N. et al. Quantification of mtDNA in single oocytes, polar bodies and subcellular components by real-time rapid cycle fluorescence monitored PCR. Zygote 8, 209–215 (2000).
Gammage, P.A. et al. Near-complete elimination of mutant mtDNA by iterative or dynamic dose-controlled treatment with mtZFNs. Nucleic Acids Res. 44, 7804–7816 (2016).
Jo, A. et al. Efficient mitochondrial genome editing by CRISPR/Cas9. BioMed Res. Int. 2015, 305716 (2015).
Katayama, M. et al. Mitochondrial distribution and microtubule organization in fertilized and cloned porcine embryos: implications for developmental potential. Dev. Biol. 299, 206–220 (2006).
Shoji, S. et al. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 25, 834–845 (2006).
Koutsopoulos, O.S. et al. Human Miltons associate with mitochondria and induce microtubule-dependent remodeling of mitochondrial networks. Biochim. Biophys. Acta 1803, 564–574 (2010).
Azoury, J. et al. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr. Biol. 18, 1514–1519 (2008).
Chaigne, A. et al. F-actin mechanics control spindle centring in the mouse zygote. Nat. Commun. 7, 10253 (2016).
Hikabe, O. et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 539, 299–303 (2016).
Ma, H. et al. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature 524, 234–238 (2015).
Cross, P.C. & Brinster, R.L. In vitro development of mouse oocytes. Biol. Reprod. 3, 298–307 (1970).
Cha, K.Y. et al. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil. Steril. 55, 109–113 (1991).
Bredenoord, A.L. & Hyun, I. Ethics of stem cell-derived gametes made in a dish: fertility for everyone? EMBO Mol. Med. 9, 396–398 (2017).
Cohen, I.G., Daley, G.Q. & Adashi, E.Y. Disruptive reproductive technologies. Sci. Transl. Med. 9, eaag2959 (2017).
Palacios-González, C. Mitochondrial replacement techniques: egg donation, genealogy and eugenics. Monash Bioeth. Rev. 34, 37–51 (2016).
Appleby, J.B., Scott, R. & Wilkinson, S. The ethics of mitochondrial replacement. Bioethics 31, 2–6 (2017).
Nuffield Council on Bioethics. Novel techniques for the prevention of mitochondrial DNA disorders: an ethical review http://nuffieldbioethics.org/project/mitochondrial-dna-disorders (2012).
Gleicher, N., Kushnir, V., Albertini, D. & Barad, D. First birth following spindle cell transfer. Reprod. Biomed. Online https://dx.doi.org/10.1016/j.rbmo.2017.07.006 (2017).
Liao, S.M. Do mitochondrial replacement techniques affect qualitative or numerical identity? Bioethics 31, 20–26 (2017).
Newson, A.J. & Wrigley, A. Is mitochondrial donation germ-line gene therapy? classifications and ethical implications. Bioethics 31, 55–67 (2017).
Bredenoord, A.L. & Appleby, J.B. Mitochondrial replacement techniques: remaining ethical challenges. Cell Stem Cell 21, 301–304 (2017).
Wallace, D.C. & Chalkia, D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb. Perspect. Biol. 5, a021220 (2013).
Thorburn, D., Wilton, L. & Stock-Myers, S. Healthy baby girl born following pre-implantation genetic diagnosis for mitochondrial DNA m.8993t>g mutation. Mol. Genet. Metab. 98, 5–6 (2009).
Monnot, S. et al. Segregation of mtDNA throughout human embryofetal development: m.3243A>G as a model system. Hum. Mutat. 32, 116–125 (2011).
Treff, N.R. et al. Blastocyst preimplantation genetic diagnosis (PGD) of a mitochondrial DNA disorder. Fertil. Steril. 98, 1236–1240 (2012).
Sallevelt, S.C. et al. Preimplantation genetic diagnosis in mitochondrial DNA disorders: challenge and success. J. Med. Genet. 50, 125–132 (2013).
Steffann, J. et al. Data from artificial models of mitochondrial DNA disorders are not always applicable to humans. Cell Rep. 7, 933–934 (2014).
Heindryckx, B. et al. Mutation-free baby born from a mitochondrial encephalopathy, lactic acidosis and stroke-like syndrome carrier after blastocyst trophectoderm preimplantation genetic diagnosis. Mitochondrion 18, 12–17 (2014).
Kondoh, H., Lleonart, M.E., Bernard, D. & Gil, J. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histol. Histopathol. 22, 85–90 (2007).
Bernstein, B.E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Tucker, K.L., Talbot, D., Lee, M.A., Leonhardt, H. & Jaenisch, R. Complementation of methylation deficiency in embryonic stem cells by a DNA methyltransferase minigene. Proc. Natl. Acad. Sci. USA 93, 12920–12925 (1996).
Eggan, K. et al. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc. Natl. Acad. Sci. USA 98, 6209–6214 (2001).
Matilainen, O., Quirós, P.M. & Auwerx, J. Mitochondria and epigenetics - crosstalk in homeostasis and stress. Trends Cell Biol. 27, 453–463 (2017).
Yang, V.S. et al. Geminin escapes degradation in G1 of mouse pluripotent cells and mediates the expression of Oct4, Sox2, and Nanog. Curr. Biol. 21, 692–699 (2011).
Mishra, P. & Chan, D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 15, 634–646 (2014).
Lonergan, T., Bavister, B. & Brenner, C. Mitochondria in stem cells. Mitochondrion 7, 289–296 (2007).
Prigione, A. & Adjaye, J. Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int. J. Dev. Biol. 54, 1729–1741 (2010).
Deglincerti, A., Etoc, F., Ozair, M.Z. & Brivanlou, A.H. Self-organization of spatial patterning in human embryonic stem cells. Curr. Top. Dev. Biol. 116, 99–113 (2016).
Shahbazi, M.N. et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 18, 700–708 (2016).
Acknowledgements
A.C.F.P. is grateful for support from the Medical Research Council, UK (grants MR/N000080/1 and MR/N020294/1). The authors thank Rob Taylor, Charlotte Alston, Emma Watson, Sam Byerley, Jane Stewart and Robert McFarland (Wellcome Centre for Mitochondrial Research Newcastle University and Newcastle upon Tyne Hospitals NHS Foundation Trust) for unpublished data included in Table 2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Greenfield, A., Braude, P., Flinter, F. et al. Assisted reproductive technologies to prevent human mitochondrial disease transmission. Nat Biotechnol 35, 1059–1068 (2017). https://doi.org/10.1038/nbt.3997
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3997
This article is cited by
-
Spatiotemporal Distribution and Function of Mitochondria in Oocytes
Reproductive Sciences (2024)
-
Autologous non-invasively derived stem cells mitochondria transfer shows therapeutic advantages in human embryo quality rescue
Biological Research (2023)
-
Glucocorticoid receptor modulates myeloid-derived suppressor cell function via mitochondrial metabolism in immune thrombocytopenia
Cellular & Molecular Immunology (2022)
-
Mitochondria: emerging therapeutic strategies for oocyte rescue
Reproductive Sciences (2022)
-
Reduction of mtDNA heteroplasmy in mitochondrial replacement therapy by inducing forced mitophagy
Nature Biomedical Engineering (2022)