Abstract
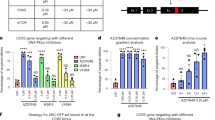
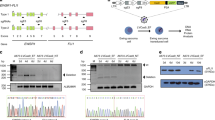
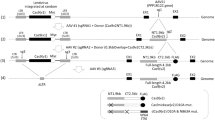
Specifically targeting genomic rearrangements and mutations in tumor cells remains an elusive goal in cancer therapy. Here, we used Cas9-based genome editing to introduce the gene encoding the prodrug-converting enzyme herpes simplex virus type 1 thymidine kinase (HSV1-tk) into the genomes of cancer cells carrying unique sequences resulting from genome rearrangements. Specifically, we targeted the breakpoints of TMEM135–CCDC67 and MAN2A1–FER fusions in human prostate cancer or hepatocellular carcinoma cells in vitro and in mouse xenografts. We designed one adenovirus to deliver the nickase Cas9D10A and guide RNAs targeting the breakpoint sequences, and another to deliver an EGFP-HSV1-tk construct flanked by sequences homologous to those surrounding the breakpoint. Infection with both viruses resulted in breakpoint-dependent expression of EGFP-tk and ganciclovir-mediated apoptosis. When mouse xenografts were treated with adenoviruses and ganciclovir, all animals showed decreased tumor burden and no mortality during the study. Thus, Cas9-mediated suicide-gene insertion may be a viable genotype-specific cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hanahan, D. & Weinberg, R.A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Yu, Y.P. et al. Novel fusion transcripts associate with progressive prostate cancer. Am. J. Pathol. 184, 2840–2849 (2014).
Mojica, F.J., Díez-Villaseñor, C., García-Martínez, J. & Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 60, 174–182 (2005).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Esvelt, K.M., Smidler, A.L., Catteruccia, F. & Church, G.M. Concerning RNA-guided gene drives for the alteration of wild populations. eLife 3, 03401 (2014).
Ran, F.A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Smith, K.O., Galloway, K.S., Kennell, W.L., Ogilvie, K.K. & Radatus, B.K. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob. Agents Chemother. 22, 55–61 (1982).
Van Rompay, A.R., Johansson, M. & Karlsson, A. Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases. Pharmacol. Ther. 87, 189–198 (2000).
Yu, Y.P. et al. Genomic copy number variations in the genomes of leukocytes predict prostate cancer clinical outcomes. PLoS One 10, e0135982 (2015).
Luo, J.H. et al. Discovery and classification of fusion transcripts in prostate cancer and normal prostate tissue. Am. J. Pathol. 185, 1834–1845 (2015).
Ohnuki, Y., Marnell, M.M., Babcock, M.S., Lechner, J.F. & Kaighn, M.E. Chromosomal analysis of human prostatic adenocarcinoma cell lines. Cancer Res. 40, 524–534 (1980).
Bernardino, J. et al. Characterization of chromosome changes in two human prostatic carcinoma cell lines (PC-3 and DU145) using chromosome painting and comparative genomic hybridization. Cancer Genet. Cytogenet. 96, 123–128 (1997).
Chen, Z.H. et al. MAN2A1-FER fusion gene is expressed by human liver and other tumor types and has oncogenic activity in mice. Gastroenterology http://dx.doi.org/10.1053/j.gastro.2016.12.036 (2017).
Hanahan, D. & Weinberg, R.A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Yu, C. et al. Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell 16, 142–147 (2015).
Hsu, P.D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Kozarsky, K.F. & Wilson, J.M. Gene therapy: adenovirus vectors. Curr. Opin. Genet. Dev. 3, 499–503 (1993).
Anderson, R.D., Haskell, R.E., Xia, H., Roessler, B.J. & Davidson, B.L. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 7, 1034–1038 (2000).
Wang, H. et al. p53-induced gene 3 mediates cell death induced by glutathione peroxidase 3. J. Biol. Chem. 287, 16890–16902 (2012).
Zhu, Z.H. et al. Integrin alpha 7 interacts with high temperature requirement A2 (HtrA2) to induce prostate cancer cell death. Am. J. Pathol. 177, 1176–1186 (2010).
Luo, K.L., Luo, J.H. & Yu, Y.P. (−)-Epigallocatechin-3-gallate induces Du145 prostate cancer cell death via downregulation of inhibitor of DNA binding 2, a dominant negative helix-loop-helix protein. Cancer Sci. 101, 707–712 (2010).
Han, Y.C. et al. Interaction of integrin-linked kinase and miniature chromosome maintenance 7-mediating integrin alpha7 induced cell growth suppression. Cancer Res. 70, 4375–4384 (2010).
Zhu, Z.H., Yu, Y.P., Shi, Y.K., Nelson, J.B. & Luo, J.H. CSR1 induces cell death through inactivation of CPSF3. Oncogene 28, 41–51 (2009).
Shi, Y.K., Yu, Y.P., Tseng, G.C. & Luo, J.H. Inhibition of prostate cancer growth and metastasis using small interference RNA specific for minichromosome complex maintenance component 7. Cancer Gene Ther. 17, 694–699 (2010).
Yu, Y.P. et al. Glutathione peroxidase 3, deleted or methylated in prostate cancer, suppresses prostate cancer growth and metastasis. Cancer Res. 67, 8043–8050 (2007).
Ren, B. et al. Analysis of integrin alpha7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcoma. J. Natl. Cancer Inst. 99, 868–880 (2007).
Yu, G. et al. CSR1 suppresses tumor growth and metastasis of prostate cancer. Am. J. Pathol. 168, 597–607 (2006).
Maruyama, T. et al. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 33, 538–542 (2015).
Han, Y.C. et al. Metallothionein 1 h tumour suppressor activity in prostate cancer is mediated by euchromatin methyltransferase 1. J. Pathol. 230, 184–193 (2013).
Ren, B. et al. MCM7 amplification and overexpression are associated with prostate cancer progression. Oncogene 25, 1090–1098 (2006).
Jing, L. et al. Expression of myopodin induces suppression of tumor growth and metastasis. Am. J. Pathol. 164, 1799–1806 (2004).
Demetris, A.J., Seaberg, E.C., Wennerberg, A., Ionellie, J. & Michalopoulos, G. Ductular reaction after submassive necrosis in humans: special emphasis on analysis of ductular hepatocytes. Am. J. Pathol. 149, 439–448 (1996).
Yu, Y.P. et al. Whole-genome methylation sequencing reveals distinct impact of differential methylations on gene transcription in prostate cancer. Am. J. Pathol. 183, 1960–1970 (2013).
Lin, F. et al. Myopodin, a synaptopodin homologue, is frequently deleted in invasive prostate cancers. Am. J. Pathol. 159, 1603–1612 (2001).
Acknowledgements
We thank S. Zheng for technical support. This work was supported by grants from the National Cancer Institute to JHL (RO1 CA098249 to J.-H.L.), the Department of Defense (W81XWH-16-1-0364) to J.-H.L. and the University of Pittsburgh Cancer Institute to J.-H.L., G.K.M. and J.B.N.
Author information
Authors and Affiliations
Contributions
J.-H.L. and Y.P.Y. conceived the concept of the project and devised the research strategy. Z.-H.C. and Z.-H.Z. performed most experiments, S.M. provided materials, G.K.M. and J.B.N. provided expertise and advice on the biology of and therapies for liver cancer and prostate cancer. S.L. and G.T. performed biostatistics and bioinformatics analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Tables 1–7 (PDF 1593 kb)
Rights and permissions
About this article
Cite this article
Chen, ZH., Yu, Y., Zuo, ZH. et al. Targeting genomic rearrangements in tumor cells through Cas9-mediated insertion of a suicide gene. Nat Biotechnol 35, 543–550 (2017). https://doi.org/10.1038/nbt.3843
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3843
This article is cited by
-
Recent Advances and Therapeutic Strategies Using CRISPR Genome Editing Technique for the Treatment of Cancer
Molecular Biotechnology (2023)
-
In vivo genome editing in mouse restores dystrophin expression in Duchenne muscular dystrophy patient muscle fibers
Genome Medicine (2021)
-
Delivery of cancer therapies by synthetic and bio-inspired nanovectors
Molecular Cancer (2021)
-
Review of applications of CRISPR-Cas9 gene-editing technology in cancer research
Biological Procedures Online (2021)
-
Detection of fusion gene transcripts in the blood samples of prostate cancer patients
Scientific Reports (2021)