Abstract
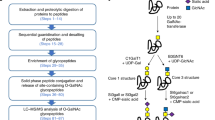
Comprehensive characterization of protein glycosylation is critical for understanding the structure and function of glycoproteins. However, due to the complexity and heterogeneity of glycoprotein conformations, current glycoprotein analyses focus mainly on either the de-glycosylated glycosylation site (glycosite)-containing peptides or the released glycans. Here, we describe a chemoenzymatic method called solid phase extraction of N-linked glycans and glycosite-containing peptides (NGAG) for the comprehensive characterization of glycoproteins that is able to determine glycan heterogeneity for individual glycosites in addition to providing information about the total N-linked glycan, glycosite-containing peptide and glycoprotein content of complex samples. The NGAG method can also be applied to quantitatively detect glycoprotein alterations in total and site-specific glycan occupancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Moremen, K.W., Tiemeyer, M. & Nairn, A.V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 13, 448–462 (2012).
Nothaft, H. & Szymanski, C.M. Protein glycosylation in bacteria: sweeter than ever. Nat. Rev. Microbiol. 8, 765–778 (2010).
Drake, P.M. et al. Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clin. Chem. 56, 223–236 (2010).
Zhang, H., Li, X.J., Martin, D.B. & Aebersold, R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat. Biotechnol. 21, 660–666 (2003).
Kaji, H. et al. Lectin affinity capture, isotope-coded tagging and mass spectrometry to identify N-linked glycoproteins. Nat. Biotechnol. 21, 667–672 (2003).
Nilsson, J. et al. Enrichment of glycopeptides for glycan structure and attachment site identification. Nat. Methods 6, 809–811 (2009).
Jensen, P.H., Karlsson, N.G., Kolarich, D. & Packer, N.H. Structural analysis of N- and O-glycans released from glycoproteins. Nat. Protoc. 7, 1299–1310 (2012).
Wada, Y., Tajiri, M. & Yoshida, S. Hydrophilic affinity isolation and MALDI multiple-stage tandem mass spectrometry of glycopeptides for glycoproteomics. Anal. Chem. 76, 6560–6565 (2004).
Yu, L., Li, X., Guo, Z., Zhang, X. & Liang, X. Hydrophilic interaction chromatography based enrichment of glycopeptides by using click maltose: a matrix with high selectivity and glycosylation heterogeneity coverage. Chem. Eur. J. 15, 12618–12626 (2009).
Paxton, R.J., Mooser, G., Pande, H., Lee, T.D. & Shively, J.E. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc. Natl. Acad. Sci. USA 84, 920–924 (1987).
Liu, T. et al. Capillary electrophoresis-electrospray mass spectrometry for the characterization of high-mannose-type N-glycosylation and differential oxidation in glycoproteins by charge reversal and protease/glycosidase digestion. Anal. Chem. 73, 5875–5885 (2001).
Chen, S. et al. Multiplexed analysis of glycan variation on native proteins captured by antibody microarrays. Nat. Methods 4, 437–444 (2007).
Zielinska, D.F., Gnad, F., Wis´niewski, J.R. & Mann, M. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 141, 897–907 (2010).
Zhang, W., Wang, H., Tang, H. & Yang, P. Endoglycosidase-mediated incorporation of 18O into glycans for relative glycan quantitation. Anal. Chem. 83, 4975–4981 (2011).
Shah, P. et al. Mass spectrometric analysis of sialylated glycans with use of solid-phase labeling of sialic acids. Anal. Chem. 85, 3606–3613 (2013).
Breidenbach, M.A. et al. Targeted metabolic labeling of yeast N-glycans with unnatural sugars. Proc. Natl. Acad. Sci. USA 107, 3988–3993 (2010).
Scott, N.E. et al. Simultaneous glycan-peptide characterization using hydrophilic interaction chromatography and parallel fragmentation by CID, higher energy collisional dissociation, and electron transfer dissociation MS applied to the N-linked glycoproteome of Campylobacter jejuni. Mol. Cell. Proteomics 10, M000031-MCP201 (2011).
Bern, M., Kil, Y.J. & Becker, C. Byonic: advanced Peptide and protein identification software. Current Protocols in Bioinformatics 13.20.11–13.20.14 (2012).
Kolarich, D., Jensen, P.H., Altmann, F. & Packer, N.H. Determination of site-specific glycan heterogeneity on glycoproteins. Nat. Protoc. 7, 1285–1298 (2012).
Lattová, E., Kapková, P., Krokhin, O. & Perreault, H. Method for investigation of oligosaccharides from glycopeptides: direct determination of glycosylation sites in proteins. Anal. Chem. 78, 2977–2984 (2006).
Pompach, P., Chandler, K.B., Lan, R., Edwards, N. & Goldman, R. Semi-automated identification of N-Glycopeptides by hydrophilic interaction chromatography, nano-reverse-phase LC-MS/MS, and glycan database search. J. Proteome Res. 11, 1728–1740 (2012).
Parker, B.L. et al. Site-specific glycan-peptide analysis for determination of N-glycoproteome heterogeneity. J. Proteome Res. 12, 5791–5800 (2013).
Yang, W. et al. Glycoform analysis of recombinant and human immunodeficiency virus envelope protein gp120 via higher energy collisional dissociation and spectral-aligning strategy. Anal. Chem. 86, 6959–6967 (2014).
Wu, S.-W., Pu, T.-H., Viner, R. & Khoo, K.-H. Novel LC-MS2 product dependent parallel data acquisition function and data analysis workflow for sequencing and identification of intact glycopeptides. Anal. Chem. 86, 5478–5486 (2014).
Wu, S.-W., Liang, S.-Y., Pu, T.-H., Chang, F.-Y. & Khoo, K.-H. Sweet-Heart - an integrated suite of enabling computational tools for automated MS2/MS3 sequencing and identification of glycopeptides. J. Proteomics 84, 1–16 (2013).
Brancia, F.L., Oliver, S.G. & Gaskell, S.J. Improved matrix-assisted laser desorption/ionization mass spectrometric analysis of tryptic hydrolysates of proteins following guanidination of lysine-containing peptides. Rapid Commun. Mass Spectrom. 14, 2070–2073 (2000).
Tian, Y., Zhou, Y., Elliott, S., Aebersold, R. & Zhang, H. Solid-phase extraction of N-linked glycopeptides. Nat. Protoc. 2, 334–339 (2007).
Toghi Eshghi, S., Shah, P., Yang, W., Li, X. & Zhang, H. GPQuest: A Spectral Library Matching Algorithm for Site-Specific Assignment of Tandem Mass Spectra to Intact N-glycopeptides. Anal. Chem. 87, 5181–5188 (2015).
Freeze, H. & Elbein, A. Essentials of Glycobiology. (eds. Varki, A. et al.) 66 (Cold Spring Harbor Laboratory Press, 2009).
Vizcaíno, J.A. et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41, D1063–D1069 (2013).
Ong, S.-E. & Mann, M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC). Nat. Protoc. 1, 2650–2660 (2006).
Sun, S., Zhou, J.-Y., Yang, W. & Zhang, H. Inhibition of protein carbamylation in urea solution using ammonium-containing buffers. Anal. Biochem. 446, 76–81 (2014).
Panchaud, A. et al. ANIBAL, stable isotope-based quantitative proteomics by aniline and benzoic acid labeling of amino and carboxylic groups. Mol. Cell. Proteomics 7, 800–812 (2008).
Schilling, O., Barré, O., Huesgen, P.F. & Overall, C.M. Proteome-wide analysis of protein carboxy termini: C terminomics. Nat. Methods 7, 508–511 (2010).
Yang, S.J., Li, Y., Shah, P.K. & Zhang, H. Glycomic analysis using glycoprotein immobilization for glycan extraction. Anal. Chem. 85, 5555–5561 (2013).
Sun, S. et al. Analysis of N-glycoproteins using genomic N-glycosite prediction. J. Proteome Res. 12, 5609–5615 (2013).
Hägglund, P., Bunkenborg, J., Elortza, F., Jensen, O.N. & Roepstorff, P. A new strategy for identification of N-glycosylated proteins and unambiguous assignment of their glycosylation sites using HILIC enrichment and partial deglycosylation. J. Proteome Res. 3, 556–566 (2004).
Yang, S.J. & Zhang, H. Glycan analysis by reversible reaction to hydrazide beads and mass spectrometry. Anal. Chem. 84, 2232–2238 (2012).
Ceroni, A. et al. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 7, 1650–1659 (2008).
Pruitt, K.D., Tatusova, T. & Maglott, D.R. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 35, D61–D65 (2007).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Keller, A., Eng, J., Zhang, N., Li, X.J. & Aebersold, R. A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Mol. Syst. Biol. 1, 0017 (2005).
Acknowledgements
This work was supported by the National Institutes of Health, National Cancer Institute, Clinical Proteomic Tumor Analysis Consortium (U24CA160036), the Early Detection Research Network (EDRN, U01CA152813 and U24CA115102) and R01CA112314, and by the National Institutes of Health, National Heart, Lung, and Blood Institute Programs of Excellence in Glycosciences (PEG, P01HL107153) and the Johns Hopkins Proteomics Center (N01-HV-00240).
Author information
Authors and Affiliations
Contributions
S.S. and H.Z. prepared the manuscript with contributions from all co-authors; S.S. conducted most experimental and data analyses with support from other co-authors; P.S. performed part of mass spectrometric analyses; P.S. and W.Y. contributed to part of data analyses; S.T.E. developed software for intact glycopeptide analysis; N.T. and N.H. cultured cells; S.Y. and P.A. contributed to part of the glycan analysis experiments; L.C. assisted in some of sample preparation experiments; D.W.C. and Z.Z. provided additional research support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 The timeline of the NGAG method for extraction of N-linked glycans and glycosite-containing peptides.
The timeline can be changed based on the number of samples.
Supplementary Figure 2 Identification of N-linked glycans, glycosite-containing peptides, and intact glycopeptides from bovine fetuin using the NGAG method.
(a) MALDI-TOF-MS spectrum of fetuin glycans. The possible glycan structures of the top 13 peaks are shown in the spectrum. (b) MS/MS spectrum of a glycosite (Asn156)-containing fetuin peptide. (c) MS/MS spectrum of an intact glycopeptide at glycosite Asn156 with a HexNAc5Hex6 (N5H6) glycan attached. Oxonium ions (highlighted in green) were used to select the glycopeptide spectra from the global data, and the accurate masses of the precursor ion and peptide+HexNAc/peptide (highlighted in blue) fragment ions as well as b/y fragment ions were used to identify the intact glycopeptide. (d) Site-specific heterogeneity of bovine fetuin. N: HexNAc; H: Hexose; F: Fucose; A: Sialic acid. The intact glycopeptides were identified from triplicate LC-MS/MS analysis.
Supplementary Figure 3 MS/MS spectra of the glycosite-containing peptides (deglycosylated peptides) identified from bovine fetuin.
(a) The peptide N#CSVR with glycosite 99. (b) The peptide N#DSR with glycosite 156. “.” indicates the cleavage site by trypsin. The LC-MS/MS data was analyzed by Proteome Discoverer software (Thermo Fisher Scientific) allowing trypsin (semi) as the enzyme and at least 4 amino acids for peptide identification.
Supplementary Figure 5 The detection of glycans with 1Da mass difference by LC-MS using a Q-Exactive mass spectrometer.
Two pairs of glycans with 1Da mass difference were used as the examples. These glycans with 1Da differences can be clearly distinguished based on their isotopic masses and retention time (which are mainly determined by the number of aniline-modified sialic acids) in LC-MS analysis.
Supplementary Figure 7 Reproducible analyses of N-glycans and glycosites using the NGAG method.
(a) The number of identified glycans from three NGAG isolations. Each glycan sample was analyzed once by LC-MS/MS. (b) Label-free quantification of the identified glycans between two isolations from the same amount of fetuin. (c) Relative quantification of glycosite-containing peptides (iTRAQ labeling method) isolated from different amounts of fetuin. (d) Label-free quantification of intact glycopeptides between replicate analyses of tryptic peptides from fetuin. (e) Comparison of identified OVCAR-3 cell glycans among three NGAG isolations. (f) Comparison of identified glycosite-containing peptides from OVCAR-3 cells among three NGAG isolations. (g) Comparison of identified glycosite-containing peptides among three LC-MS/MS injection of the same sample isolated from OVCAR-3 cells. Each glycan or glycosite-containing peptide sample was analyzed once by LC-MS/MS.
Supplementary Figure 8 Liquid chromatography profiles of the glycans of OVCAR-3 cells from triplicate isolations using the NGAG method.
The base peak profiles of the raw files of the triplicate isolations are displayed in Xcalibur.
Supplementary Figure 9 Liquid chromatography profiles of glycosite-containing peptides of OVCAR-3 cells from (a) 3 LC-MS/MS replicates and (b) 3 isolation replicates using the NGAG method.
The base peak profiles of the raw files of triplicate isolations are displayed in Xcalibur.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 4044 kb)
Supplementary Text and Figures
Supplementary Results and Discussion, Supplementary Figures 1–9 and Supplementary Table 8 (PDF 2500 kb)
Supplementary Table 1
N-linked glycan compositions detected from bovine fetuin using NGAG method (XLSX 21 kb)
Supplementary Table 2
Intact N-glycopeptides identified from the global data of bovine fetuin (XLSX 24 kb)
Supplementary Table 3
N-linked glycan compositions detected from OVCAR-3 cells using NGAG method (XLSX 20 kb)
Supplementary Table 4
N-Linked glycosite-containing peptides identified from OVCAR-3 cells using NGAG method (XLSX 207 kb)
Supplementary Table 5
N-Linked glycosite-containing peptides identified from OVCAR-3 cells using SPEG method (XLSX 137 kb)
Supplementary Table 6
All N-linked glycosite-containing peptides identified from OVCAR-3 cells using NGAG and SPEG methods (XLSX 395 kb)
Supplementary Table 7
Intact N-glycopeptides identified from HILIC enriched glycopeptides from OVCAR-3 cells (1X FDR) (XLSX 435 kb)
Rights and permissions
About this article
Cite this article
Sun, S., Shah, P., Eshghi, S. et al. Comprehensive analysis of protein glycosylation by solid-phase extraction of N-linked glycans and glycosite-containing peptides. Nat Biotechnol 34, 84–88 (2016). https://doi.org/10.1038/nbt.3403
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3403
This article is cited by
-
Characterization of aberrant glycosylation associated with osteoarthritis based on integrated glycomics methods
Arthritis Research & Therapy (2023)
-
Cyst fluid glycoproteins accurately distinguishing malignancies of pancreatic cystic neoplasm
Signal Transduction and Targeted Therapy (2023)
-
Maximal performance of intact N-glycopeptide enrichment using sequential HILIC and MAX columns
Analytical and Bioanalytical Chemistry (2023)
-
Revealing the human mucinome
Nature Communications (2022)
-
pGlycoQuant with a deep residual network for quantitative glycoproteomics at intact glycopeptide level
Nature Communications (2022)