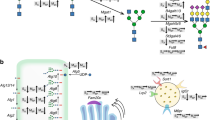
Abstract
Production of glycoprotein therapeutics in Chinese hamster ovary (CHO) cells is limited by the cells' generic capacity for N-glycosylation, and production of glycoproteins with desirable homogeneous glycoforms remains a challenge. We conducted a comprehensive knockout screen of glycosyltransferase genes controlling N-glycosylation in CHO cells and constructed a design matrix that facilitates the generation of desired glycosylation, such as human-like α2,6-linked sialic acid capping. This engineering approach will aid the production of glycoproteins with improved properties and therapeutic potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Walsh, G. Nat. Biotechnol. 32, 992–1000 (2014).
Walsh, G. Drug Discov. Today 15, 773–780 (2010).
Sasaki, H., Ochi, N., Dell, A. & Fukuda, M. Biochemistry 27, 8618–8626 (1988).
Sinclair, A.M. & Elliott, S. J. Pharm. Sci. 94, 1626–1635 (2005).
Patnaik, S.K. & Stanley, P. Methods Enzymol. 416, 159–182 (2006).
Yamane-Ohnuki, N. et al. Biotechnol. Bioeng. 87, 614–622 (2004).
Malphettes, L. et al. Biotechnol. Bioeng. 106, 774–783 (2010).
Steentoft, C. et al. Glycobiology 24, 663–680 (2014).
Xu, X. et al. Nat. Biotechnol. 29, 735–741 (2011).
Lewis, N.E. et al. Nat. Biotechnol. 31, 759–765 (2013).
North, S.J. et al. J. Biol. Chem. 285, 5759–5775 (2010).
Amado, M., Almeida, R., Schwientek, T. & Clausen, H. Biochim. Biophys. Acta 1473, 35–53 (1999).
Narimatsu, H. Curr. Opin. Struct. Biol. 16, 567–575 (2006).
Fukuda, M.N., Sasaki, H., Lopez, L. & Fukuda, M. Blood 73, 84–89 (1989).
Maresca, M., Lin, V.G., Guo, N. & Yang, Y. Genome Res. 23, 539–546 (2013).
El Maï, N., Donadio-Andrei, S., Iss, C., Calabro, V. & Ronin, C. Methods Mol. Biol. 988, 19–29 (2013).
DeFrees, S. et al. Glycobiology 16, 833–843 (2006).
Hamilton, S.R. et al. Science 313, 1441–1443 (2006).
Duda, K. et al. Nucleic Acids Res. 42, e84 (2014).
Zhang, Y. et al. Nucleic Acids Res. 43, e59 (2015).
Acknowledgements
We would like to express our sincere gratitude to the SAFC Sigma team including K. Kayser and N. Sealover for their help with ZFN targeting constructs. We are also grateful to B. Palsson for help throughout this work and critical comments on the manuscript. We are grateful to M. Uhlen, L.E. Pedersen and B. Voldborg at the Novo Nordisk Foundation Center for Biosustainability, Danish Technical University, for RNA-seq analysis. This work was supported by the Novo Nordisk Foundation, Kirsten og Freddy Johansen Fonden, The Mizutani Foundation, A.P. Møller og Hustru Chastine Mc-Kinney Møllers Fond til Almene Formaal, Copenhagen University Excellence Programme for Interdisciplinary Research (CDO2016), the Danish National Research Foundation (DNRF107) and The Danish Councils for Strategic and Independent Research. All reagents and cell lines used in the study are available upon request for research purposes under a material transfer agreement.
Author information
Authors and Affiliations
Contributions
Z.Y. designed, planned and ran most of the ZFN targeting experiments and co-wrote the manuscript. S.W. performed purification of EPO and IgG. A.H. performed the glycoprofiling. M.A.S. contributed to expression of EPO and IgG. M.F. contributed to ZFN design. S.H.R., C.B. and M.B.V.-C. contributed to the design of experiments for homogeneous glycoPEGylation of monoantennary N-glycans. S.Y.V., E.P.B., C.K. and H.H.W. contributed to the design of experiments. H.C. designed, planned and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent application has been filed on which some of the authors are listed as inventors.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 (PDF 3698 kb)
Supplementary Additional Spectra Combined PDF
Supplementary Spectra 1–38 and Supplementary Tables 1–4 (PDF 5460 kb)
Rights and permissions
About this article
Cite this article
Yang, Z., Wang, S., Halim, A. et al. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat Biotechnol 33, 842–844 (2015). https://doi.org/10.1038/nbt.3280
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3280
This article is cited by
-
From Efficiency to Yield: Exploring Recent Advances in CHO Cell Line Development for Monoclonal Antibodies
Molecular Biotechnology (2024)
-
Glycoengineered keratinocyte library reveals essential functions of specific glycans for all stages of HSV-1 infection
Nature Communications (2023)
-
Identification of global inhibitors of cellular glycosylation
Nature Communications (2023)
-
Successive remodeling of IgG glycans using a solid-phase enzymatic platform
Communications Biology (2022)
-
Antibody-dependent cellular cytotoxicity-null effector developed using mammalian and plant GlycoDelete platform
Scientific Reports (2022)