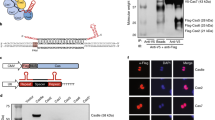
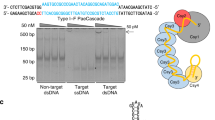
Abstract
Prokaryotic type II CRISPR-Cas systems can be adapted to enable targeted genome modifications across a range of eukaryotes1,2,3,4,5,6,7. Here we engineer this system to enable RNA-guided genome regulation in human cells by tethering transcriptional activation domains either directly to a nuclease-null Cas9 protein or to an aptamer-modified single guide RNA (sgRNA). Using this functionality we developed a transcriptional activation–based assay to determine the landscape of off-target binding of sgRNA:Cas9 complexes and compared it with the off-target activity of transcription activator–like (TALs) effectors8,9. Our results reveal that specificity profiles are sgRNA dependent, and that sgRNA:Cas9 complexes and 18-mer TAL effectors can potentially tolerate 1–3 and 1–2 target mismatches, respectively. By engineering a requirement for cooperativity through offset nicking for genome editing or through multiple synergistic sgRNAs for robust transcriptional activation, we suggest methods to mitigate off-target phenomena. Our results expand the versatility of the sgRNA:Cas9 tool and highlight the critical need to engineer improved specificity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cho, S.W., Kim, S., Kim, J.M. & Kim, J.S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
DiCarlo, J.E. et al. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41, 4336–4343 (2013).
Hwang, W.Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013).
Jinek, M. et al. RNA-programmed genome editing in human cells. eLife 2, e00471 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Qi, L.S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Boch, J. et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512 (2009).
Moscou, M.J. & Bogdanove, A.J. A simple cipher governs DNA recognition by TAL effectors. Science 326, 1501 (2009).
Deltcheva, E. et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471, 602–607 (2011).
Gasiunas, G., Barrangou, R., Horvath, P. & Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 109, E2579–E2586 (2012).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Sapranauskas, R. et al. The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res. 39, 9275–9282 (2011).
Bhaya, D., Davison, M. & Barrangou, R. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 45, 273–297 (2011).
Zhang, F. et al. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat. Biotechnol. 29, 149–153 (2011).
Fusco, D. et al. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr. Biol. 13, 161–167 (2003).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Maeder, M.L. et al. Robust, synergistic regulation of human gene expression using TALE activators. Nat. Methods 10, 243–245 (2013).
Perez-Pinera, P. et al. Synergistic and tunable human gene activation by combinations of synthetic transcription factors. Nat. Methods 10, 239–242 (2013).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Patwardhan, R.P. et al. Massively parallel functional dissection of mammalian enhancers in vivo. Nat. Biotechnol. 30, 265–270 (2012).
Sanjana, N.E. et al. A transcription activator-like effector toolbox for genome engineering. Nat. Protoc. 7, 171–192 (2012).
Meckler, J.F. et al. Quantitative analysis of TALE-DNA interactions suggests polarity effects. Nucleic Acids Res. 41, 4118–4128 (2013).
Reyon, D. et al. FLASH assembly of TAL effectors for high-throughput genome editing. Nat. Biotechnol. 30, 460–465 (2012).
Streubel, J., Blucher, C., Landgraf, A. & Boch, J. TAL effector RVD specificities and efficiencies. Nat. Biotechnol. 30, 593–595 (2012).
Porteus, M.H. & Carroll, D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 23, 967–973 (2005).
Pattanayak, V., Ramirez, C.L., Joung, J.K. & Liu, D.R. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat. Methods 8, 765–770 (2011).
Gabriel, R. et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat. Biotechnol. 29, 816–823 (2011).
Certo, M.T. et al. Tracking genome engineering outcome at individual DNA breakpoints. Nat. Methods 8, 671–676 (2011).
Symington, L.S. & Gautier, J. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 45, 247–271 (2011).
Acknowledgements
P.M. thanks R. Kalhor for insightful discussions. This work was supported by US National Institutes of Health grant P50 HG005550 and Department of Energy grant DE-FG02-02ER63445.
Author information
Authors and Affiliations
Contributions
P.M., J.A. and G.M.C. conceived the study. P.M. designed and performed experiments. J.A. designed and performed bioinformatic analyses. M.M. performed experiments. P.B.S., K.M.E., S.K. and L.Y. developed reagents and performed analyses. P.M., J.A. and G.M.C. wrote the manuscript with support from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–17, Supplementary Tables 1–3 and Supplementary Notes 1–3 (PDF 28595 kb)
Rights and permissions
About this article
Cite this article
Mali, P., Aach, J., Stranges, P. et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31, 833–838 (2013). https://doi.org/10.1038/nbt.2675
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2675
This article is cited by
-
Efficient and safe therapeutic use of paired Cas9-nickases for primary hyperoxaluria type 1
EMBO Molecular Medicine (2024)
-
Targeted DNA integration in human cells without double-strand breaks using CRISPR-associated transposases
Nature Biotechnology (2024)
-
Bioinformatic and literature assessment of toxicity and allergenicity of a CRISPR-Cas9 engineered gene drive to control Anopheles gambiae the mosquito vector of human malaria
Malaria Journal (2023)
-
CRISPR/Cas9 assisted stem cell therapy in Parkinson's disease
Biomaterials Research (2023)
-
Removal of a partial genomic duplication restores synaptic transmission and behavior in the MyosinVA mutant mouse Flailer
BMC Biology (2023)