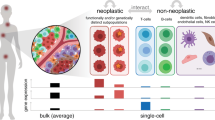
Abstract
In recent years, major advances in single-cell measurement systems have included the introduction of high-throughput versions of traditional flow cytometry that are now capable of measuring intracellular network activity, the emergence of isotope labels that can enable the tracking of a greater variety of cell markers and the development of super-resolution microscopy techniques that allow measurement of RNA expression in single living cells. These technologies will facilitate our capacity to catalog and bring order to the inherent diversity present in cancer cell populations. Alongside these developments, new computational approaches that mine deep data sets are facilitating the visualization of the shape of the data and enabling the extraction of meaningful outputs. These applications have the potential to reveal new insights into cancer biology at the intersections of stem cell function, tumor-initiating cells and multilineage tumor development. In the clinic, they may also prove important not only in the development of new diagnostic modalities but also in understanding how the emergence of tumor cell clones harboring different sets of mutations predispose patients to relapse or disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Spencer, S.L. & Sorger, P.K. Measuring and modeling apoptosis in single cells. Cell 144, 926–939 (2011).
Spencer, S.L. et al. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 459, 428–432 (2009).
Vincent, M. Cancer: a de-repression of a default survival program common to all cells? A life-history perspective on the nature of cancer. Bioessays 34, 72–82 (2012).
Greaves, M. & Maley, C.C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Visvader, J.E. Cells of origin in cancer. Nature 469, 314–322 (2011).
Medema, J.P. & Vermeulen, L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 474, 318–326 (2011).
Wang, X. et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 (2009).
Knoepfler, P. Journal club. A cell biologist looks at the risk and promise of a new insight into stem cells and cancer. Nature 457, 361 (2009).
Yang, J. et al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem. Biophys. 62, 221–228 (2012).
Zhang, J., Yang, P.L. & Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 9, 28–39 (2009).
Taussig, D.C. et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(-) fraction. Blood 115, 1976–1984 (2010).
Yap, T.A. et al. Intratumor heterogeneity: seeing the wood for the trees. Sci. Transl. Med. 4, 127ps10 (2012).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Longo, D.L. Tumor heterogeneity and personalized medicine. N. Engl. J. Med. 366, 956–957 (2012).
Cantor, H. et al. Characterization of subpopulations of T lymphocytes. I. Separation and functional studies of peripheral T-cells binding different amounts of fluorescent anti-Thy 1.2 (theta) antibody using a fluorescence-activated cell sorter (FACS). Cell. Immunol. 15, 180–196 (1975).
Parks, D.R. et al. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc. Natl. Acad. Sci. USA 76, 1962–1966 (1979).
Werner, M. et al. Microfluidic array cytometer based on refractive optical tweezers for parallel trapping, imaging and sorting of individual cells. Lab Chip 11, 2432–2439 (2011).
Wlodkowic, D. & Darzynkiewicz, Z. Rise of the micromachines: microfluidics and the future of cytometry. Methods Cell Biol. 102, 105–125 (2011).
Liu, A.Y., Roudier, M.P. & True, L.D. Heterogeneity in primary and metastatic prostate cancer as defined by cell surface CD profile. Am. J. Pathol. 165, 1543–1556 (2004).
Bragado, P. et al. Analysis of marker-defined HNSCC subpopulations reveals a dynamic regulation of tumor initiating properties. PLoS ONE 7, e29974 (2012).
Choijamts, B. et al. CD133+ cancer stem cell-like cells derived from uterine carcinosarcoma (malignant mixed Mullerian tumor). Stem Cells 29, 1485–1495 (2011).
Bonnet, D. & Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730–737 (1997).
Cho, R.W. & Clarke, M.F. Recent advances in cancer stem cells. Curr. Opin. Genet. Dev. 18, 48–53 (2008).
Lobo, N.A. et al. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 23, 675–699 (2007).
Nguyen, L.V. et al. Cancer stem cells: an evolving concept. Nat. Rev. Cancer 12, 133–143 (2012).
Irish, J.M. et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell 118, 217–228 (2004).
Kotecha, N. et al. Single-cell profiling identifies aberrant STAT5 activation in myeloid malignancies with specific clinical and biologic correlates. Cancer Cell 14, 335–343 (2008).
Irish, J.M. et al. B-cell signaling networks reveal a negative prognostic human lymphoma cell subset that emerges during tumor progression. Proc. Natl. Acad. Sci. USA 107, 12747–12754 (2010).
Palazzo, A.L. et al. Association of reactive oxygen species-mediated signal transduction with in vitro apoptosis sensitivity in chronic lymphocytic leukemia B cells. PLoS ONE 6, e24592 (2011).
Perez, O.D. & Nolan, G.P. Resistance is futile: assimilation of cellular machinery by HIV-1. Immunity 15, 687–690 (2001).
Krutzik, P.O. & Nolan, G.P. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A 55, 61–70 (2003).
Sachs, K. et al. Causal protein-signaling networks derived from multiparameter single-cell data. Science 308, 523–529 (2005).
Rosen, D.B. et al. Distinct patterns of DNA damage response and apoptosis correlate with Jak/Stat and PI3kinase response profiles in human acute myelogenous leukemia. PLoS ONE 5, e12405 (2010).
Krutzik, P.O. et al. High-content single-cell drug screening with phosphospecific flow cytometry. Nat. Chem. Biol. 4, 132–142 (2008).
Sachs, K. et al. Characterization of patient specific signaling via augmentation of Bayesian networks with disease and patient state nodes. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 6624–6627 (2009).
Sachs, K. et al. Learning signaling network structures with sparsely distributed data. J. Comput. Biol. 16, 201–212 (2009).
Rosen, D.B. et al. Assessing signaling pathways associated with in vitro resistance to cytotoxic agents in AML. Leuk. Res. 900–904 (2012).
Cesano, A. et al. Functional pathway analysis in acute myeloid leukemia using single cell network profiling assay: effect of specimen source (bone marrow or peripheral blood) on assay readouts. Cytometry B Clin. Cytom. 82, 158–172 (2012).
Longo, D.M. et al. Single-cell network profiling of peripheral blood mononuclear cells from healthy donors reveals age- and race-associated differences in immune signaling pathway activation. J. Immunol. 188, 1717–1725 (2012).
Yates, J.R., Ruse, C.I. & Nakorchevsky, A. Proteomics by mass spectrometry: approaches, advances, and applications. Annu. Rev. Biomed. Eng. 11, 49–79 (2009).
Bandura, D.R. et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 81, 6813–6822 (2009).
Bendall, S.C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Qiu, P. et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat. Biotechnol. 29, 886–891 (2011).
Newell, E.W. et al. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 36, 142–152 (2012).
Behbehani, G. et al. Single cell mass cytometry adapted to measurements of the cell cycle. Cytometry A (in the press).
Fienberg, H.G., Simonds. E.F. Fantl. W.J. Nolan. G.P. & Bodenmiller, B. A platinum-based covalent viability reagent for single-cell mass cytometry. Cytometry A 81, 467–475 (2012).
Hoshida, Y. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 69, 7385–7392 (2009).
Hoshida, Y. et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N. Engl. J. Med. 359, 1995–2004 (2008).
Mullighan, C.G. et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 322, 1377–1380 (2008).
Notta, F. et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature 469, 362–367 (2011).
Kapranov, P., Ozsolak, F. & Milos, P.M. Profiling of short RNAs using Helicos single-molecule sequencing. Methods Mol. Biol. 822, 219–232 (2012).
Nagalakshmi, U. et al. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science 320, 1344–1349 (2008).
Ozsolak, F. & Milos, P.M. RNA sequencing: advances, challenges and opportunities. Nat. Rev. Genet. 12, 87–98 (2011).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Ley, T.J. et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 456, 66–72 (2008).
Mardis, E.R. et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 361, 1058–1066 (2009).
Ding, L. et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481, 506–510 (2012).
Walter, M.J. et al. Clonal architecture of secondary acute myeloid leukemia. N. Engl. J. Med. 366, 1090–1098 (2012).
Anderson, K. et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 469, 356–361 (2011).
Hou, Y. et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell 148, 873–885 (2012).
Xu, X. et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell 148, 886–895 (2012).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Dalerba, P. et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat. Biotechnol. 29, 1120–1127 (2011).
Gibbs, K.D. Jr. et al. Decoupling of tumor-initiating activity from stable immunophenotype in HoxA9-Meis1-driven AML. Cell Stem Cell 10, 210–217 (2012).
Cornett, D.S. et al. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat. Methods 4, 828–833 (2007).
Ornatsky, O.I. et al. Study of cell antigens and intracellular DNA by identification of element-containing labels and metallointercalators using inductively coupled plasma mass spectrometry. Anal. Chem. 80, 2539–2547 (2008).
Steinhauser, M.L. et al. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature 481, 516–519 (2012).
Engelhard, C. Inductively coupled plasma mass spectrometry: recent trends and developments. Anal. Bioanal. Chem. 399, 213–219 (2011).
Nishiguchi, M. et al. Ion optical evaluation of a miniature double-focusing mass spectrograph. Eur. J. Mass Spectrom. (Chichester, Eng.) 14, 7–15 (2008).
Schilling, G.D. et al. Continuous simultaneous detection in mass spectrometry. Anal. Chem. 79, 7662–7668 (2007).
De Stefano, J.A. et al. Analysis of Pneumocystis carinii cyst wall. II. Sugar composition. J. Protozool. 37, 436–441 (1990).
Barnes, J.H. 4th. et al. Characterization of a focal plane camera fitted to a Mattauch-Herzog geometry mass spectrograph. 2. Use with an inductively coupled plasma. Anal. Chem. 76, 2531–2536 (2004).
Barnes, J.H. 4th. et al. Use of a novel array detector for the direct analysis of solid samples by laser ablation inductively coupled plasma sector-field mass spectrometry. J. Am. Soc. Mass Spectrom. 15, 769–776 (2004).
Barnes, J.H. 4th. et al. Characterization of a focal plane camera fitted to a Mattauch-Herzog geometry mass spectrograph. 1. Use with a glow-discharge source. Anal. Chem. 74, 5327–5332 (2002).
Zhang, D.S. et al. Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature 481, 520–524 (2012).
Lechene, C. et al. High-resolution quantitative imaging of mammalian and bacterial cells using stable isotope mass spectrometry. J. Biol. 5, 20 (2006).
Irish, J.M., Kotecha, N. & Nolan, G.P. Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nat. Rev. Cancer 6, 146–155 (2006).
Bendall, S.C. et al. A deep profiler's guide to cytometry. Trends Immunol. published online, doi:10.1016/j.it.2012.02.010 (2 April 2012).
Ghosn, E.E. et al. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 109, 5394–5398 (2012).
Tung, J.W. et al. Modern flow cytometry: a practical approach. Clin. Lab. Med. 27, 453–468 (2007).
Giesen, C. et al. Multiplexed immunohistochemical detection of tumor markers in breast cancer tissue using laser ablation inductively coupled plasma mass spectrometry. Anal. Chem. 83, 8177–8183 (2011).
Moreno-Gordaliza, E. et al. Elemental bioimaging in kidney by LA-ICP-MS as a tool to study nephrotoxicity and renal protective strategies in cisplatin therapies. Anal. Chem. 83, 7933–7940 (2011).
Acknowledgements
G.P.N. is supported by the Rachford and Carlota A. Harris Endowed Professorship and grants from U19 AI057229, P01 CA034233, HHSN272200700038C, 1R01CA130826, CIRM DR1-01477 and RB2-01592, NCI RFA CA 09-011, NHLBI-HV-10-05(2), European Commission HEALTH.2010.1.2-1, and the Bill and Melinda Gates Foundation (GF12141-137101). S.C.B. is supported by the Damon Runyon Cancer Research Foundation Fellowship (DRG-2017-09). The authors would also like to thank M. Angelo for useful discussions pertaining to the information in Table 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.P.N. is a paid consultant for, receives royalties from, or owns equity in the following companies whose products or services are directly or indirectly discussed in this article: Becton Dickinson, Nodality and DVS Sciences.
Rights and permissions
About this article
Cite this article
Bendall, S., Nolan, G. From single cells to deep phenotypes in cancer. Nat Biotechnol 30, 639–647 (2012). https://doi.org/10.1038/nbt.2283
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2283
This article is cited by
-
COSMOS: a platform for real-time morphology-based, label-free cell sorting using deep learning
Communications Biology (2023)
-
Mass cytometry reveals immune atlas of urothelial carcinoma
BMC Cancer (2022)
-
Single-cell analysis at the protein level delineates intracellular signaling dynamic during hematopoiesis
BMC Biology (2021)
-
Multiplexed live-cell profiling with Raman probes
Nature Communications (2021)
-
VoPo leverages cellular heterogeneity for predictive modeling of single-cell data
Nature Communications (2020)