Abstract
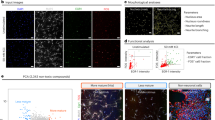
Considerable progress has been made in identifying signaling pathways that direct the differentiation of human pluripotent stem cells (hPSCs) into specialized cell types, including neurons. However, differentiation of hPSCs with extrinsic factors is a slow, step-wise process, mimicking the protracted timing of human development. Using a small-molecule screen, we identified a combination of five small-molecule pathway inhibitors that yield hPSC-derived neurons at >75% efficiency within 10 d of differentiation. The resulting neurons express canonical markers and functional properties of human nociceptors, including tetrodotoxin (TTX)-resistant, SCN10A-dependent sodium currents and response to nociceptive stimuli such as ATP and capsaicin. Neuronal fate acquisition occurs about threefold faster than during in vivo development1, suggesting that use of small-molecule pathway inhibitors could become a general strategy for accelerating developmental timing in vitro. The quick and high-efficiency derivation of nociceptors offers unprecedented access to this medically relevant cell type for studies of human pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Bystron, I., Rakic, P., Molnar, Z. & Blakemore, C. The first neurons of the human cerebral cortex. Nat. Neurosci. 9, 880–886 (2006).
Zhang, X.Q. & Zhang, S.C. Differentiation of neural precursors and dopaminergic neurons from human embryonic stem cells. Methods Mol. Biol. 584, 355–366 (2010).
Elkabetz, Y. et al. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 22, 152–165 (2008).
Perrier, A.L. et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 101, 12543–12548 (2004).
Saha, K. & Jaenisch, R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell 5, 584–595 (2009).
Chambers, S.M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Kim, D.S. et al. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. 6, 270–281 (2010).
Zhou, J. et al. High-efficiency induction of neural conversion in hESCs and hiPSCs with a single chemical inhibitor of TGF-beta superfamily receptors. Stem Cells 1741–1750 (2010).
Yu, P.B. et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat. Med. 14, 1363–1369 (2008).
Zhang, X. et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 7, 90–100 (2010).
Lee, M.K., Tuttle, J.B., Rebhun, L.I., Cleveland, D.W. & Frankfurter, A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil. Cytoskeleton 17, 118–132 (1990).
Sun, L. et al. Design, synthesis, and evaluations of substituted 3-[(3- or 4-carboxyethylpyrrol-2-yl)methylidenyl]indolin-2-ones as inhibitors of VEGF, FGF, and PDGF receptor tyrosine kinases. J. Med. Chem. 42, 5120–5130 (1999).
Bennett, C.N. et al. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 277, 30998–31004 (2002).
Dovey, H.F. et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J. Neurochem. 76, 173–181 (2001).
Gerdes, J., Schwab, U., Lemke, H. & Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 31, 13–20 (1983).
Hendzel, M.J. et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360 (1997).
Sun, Y. et al. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat. Neurosci. 11, 1283–1293 (2008).
Gerrero, M.R. et al. Brn-3.0: a POU-domain protein expressed in the sensory, immune, and endocrine systems that functions on elements distinct from known octamer motifs. Proc. Natl. Acad. Sci. USA 90, 10841–10845 (1993).
Marmigere, F. & Ernfors, P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat. Rev. Neurosci. 8, 114–127 (2007).
Papapetrou, E.P. et al. Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proc. Natl. Acad. Sci. USA 106, 12759–12764 (2009).
Aoki, Y. et al. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev. Biol. 259, 19–33 (2003).
Lee, G. et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat. Biotechnol. 25, 1468–1475 (2007).
George, L., Chaverra, M., Todd, V., Lansford, R. & Lefcort, F. Nociceptive sensory neurons derive from contralaterally migrating, fate-restricted neural crest cells. Nat. Neurosci. 10, 1287–1293 (2007).
Schlosser, G. & Northcutt, R.G. Development of neurogenic placodes in Xenopus laevis. J. Comp. Neurol. 418, 121–146 (2000).
Schlosser, G. Induction and specification of cranial placodes. Dev. Biol. 294, 303–351 (2006).
Woolf, C.J. & Ma, Q. Nociceptors–noxious stimulus detectors. Neuron 55, 353–364 (2007).
Fasano, C.A., Chambers, S.M., Lee, G., Tomishima, M.J. & Studer, L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell 6, 336–347 (2010).
Ma, Q., Fode, C., Guillemot, F. & Anderson, D.J. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 13, 1717–1728 (1999).
Marmigere, F. & Ernfors, P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat. Rev. Neurosci. 8, 114–127 (2007).
Dib-Hajj, S.D. et al. Two tetrodotoxin-resistant sodium channels in human dorsal root ganglion neurons. FEBS Lett. 462, 117–120 (1999).
Renganathan, M., Cummins, T.R. & Waxman, S.G. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J. Neurophysiol. 86, 629–640 (2001).
Jarvis, M.F. et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc. Natl. Acad. Sci. USA 99, 17179–17184 (2002).
Caterina, M.J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
North, R.A. The P2X3 subunit: a molecular target in pain therapeutics. Curr. Opin. Investig. Drugs 4, 833–840 (2003).
Kitao, Y., Robertson, B., Kudo, M. & Grant, G. Neurogenesis of subpopulations of rat lumbar dorsal root ganglion neurons including neurons projecting to the dorsal column nuclei. J. Comp. Neurol. 371, 249–257 (1996).
Dorsky, R.I., Moon, R.T. & Raible, D.W. Control of neural crest cell fate by the Wnt signalling pathway. Nature 396, 370–373 (1998).
Lee, H.Y. et al. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science 303, 1020–1023 (2004).
Cornell, R.A. & Eisen, J.S. Delta/Notch signaling promotes formation of zebrafish neural crest by repressing Neurogenin 1 function. Development 129, 2639–2648 (2002).
Molliver, D.C. et al. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19, 849–861 (1997).
Ibanez, C.F. & Ernfors, P. Hierarchical control of sensory neuron development by neurotrophic factors. Neuron 54, 673–675 (2007).
Luo, W. et al. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron 54, 739–754 (2007).
Gascon, E. et al. Hepatocyte growth factor-Met signaling is required for Runx1 extinction and peptidergic differentiation in primary nociceptive neurons. J. Neurosci. 30, 12414–12423 (2010).
Chen, C.L. et al. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron 49, 365–377 (2006).
Kramer, I. et al. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron 49, 379–393 (2006).
Yoshikawa, M. et al. Runx1 selectively regulates cell fate specification and axonal projections of dorsal root ganglion neurons. Dev. Biol. 303, 663–674 (2007).
Placantonakis, D.G. et al. BAC transgenesis in human embryonic stem cells as a novel tool to define the human neural lineage. Stem Cells 27, 521–532 (2009).
Acknowledgements
We thank J. Hendrikx (SKI Flow Cytometry Core lab), A. Viale (SKI Genomics Core lab), E. Tu (SKI stem cell facility), and M. Tomishima (SKI stem cell facility) for excellent technical support. We also thank R. McKernan for support of functional analysis, M. Postlethwaite for assistance with electrophysiology, C. Benn for help in gene expression analysis and S. Kriks for assistance with hPSC culturing. The work was supported in part through grants NS066390 from National Institute of Neurological Disorders and Stroke/US National Institutes of Health (NIH) and C026447 from New York State Stem Cell Science (NYSTEM) to L.S., R01DA024681 from the National Institute on Drug Abuse/NIH to S.-H.S., PO1NS048120 from the National Institute of Mental Health/NIH to S.-H.S., and C026399 from NYSTEM to S.M.C.
Author information
Authors and Affiliations
Contributions
S.M.C., experimental design, characterization experiments and manuscript; Y.Q., chemical screen to identify 3i; Y.M. and G.L., SOX10∷GFP BAC transgenic hPSC line generation and culturing; X.-J.Z. and L.N., initial LSB3i electrophysiology; J.B., L.C., E.S. and P.W., electrophysiology and calcium imaging experiments, PRPH characterization and manuscript; S.-H.S., electrophysiology experimental design; L.S., experimental design and manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.B., L.C., E.S. and P.W. are employees of Neusentis, Pfizer Global R&D.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1-15 and Supplementary Tables 1-2 (PDF 4200 kb)
Rights and permissions
About this article
Cite this article
Chambers, S., Qi, Y., Mica, Y. et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol 30, 715–720 (2012). https://doi.org/10.1038/nbt.2249
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2249
This article is cited by
-
An epigenetic barrier sets the timing of human neuronal maturation
Nature (2024)
-
Maximizing treatment efficacy through patient stratification in neuropathic pain trials
Nature Reviews Neurology (2023)
-
Affinity-matured DLL4 ligands as broad-spectrum modulators of Notch signaling
Nature Chemical Biology (2023)
-
Biomimetic Strategies for Peripheral Nerve Injury Repair: An Exploration of Microarchitecture and Cellularization
Biomedical Materials & Devices (2023)
-
Using induced pluripotent stem cells to investigate human neuronal phenotypes in 1q21.1 deletion and duplication syndrome
Molecular Psychiatry (2022)