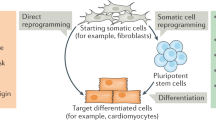
Abstract
Classic experiments such as somatic cell nuclear transfer into oocytes and cell fusion demonstrated that differentiated cells are not irreversibly committed to their fate. More recent work has built on these conclusions and discovered defined factors that directly induce one specific cell type from another, which may be as distantly related as cells from different germ layers. This suggests the possibility that any specific cell type may be directly converted into any other if the appropriate reprogramming factors are known. Direct lineage conversion could provide important new sources of human cells for modeling disease processes or for cellular-replacement therapies. For future applications, it will be critical to carefully determine the fidelity of reprogramming and to develop methods for robustly and efficiently generating human cell types of interest.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Briggs, R. & King, T.J. Transplantation of living nuclei from blastula cells into enucleated frogs' eggs. Proc. Natl. Acad. Sci. USA 38, 455–463 (1952).
Gurdon, J.B., Elsdale, T.R. & Fischberg, M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 182, 64–65 (1958).
Gurdon, J.B. From nuclear transfer to nuclear reprogramming: the reversal of cell differentiation. Annu. Rev. Cell Dev. Biol. 22, 1–22 (2006).
Gurdon, J.B. & Melton, D.A. Nuclear reprogramming in cells. Science 322, 1811–1815 (2008).
Campbell, K.H., McWhir, J., Ritchie, W.A. & Wilmut, I. Sheep cloned by nuclear transfer from a cultured cell line. Nature 380, 64–66 (1996).
Wilmut, I., Schnieke, A.E., McWhir, J., Kind, A.J. & Campbell, K.H. Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810–813 (1997).
Hochedlinger, K. & Jaenisch, R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature 415, 1035–1038 (2002).
Eggan, K. et al. Mice cloned from olfactory sensory neurons. Nature 428, 44–49 (2004).
Li, J., Ishii, T., Feinstein, P. & Mombaerts, P. Odorant receptor gene choice is reset by nuclear transfer from mouse olfactory sensory neurons. Nature 428, 393–399 (2004).
Bernstein, B.E., Meissner, A. & Lander, E.S. The mammalian epigenome. Cell 128, 669–681 (2007).
Jenuwein, T. & Allis, C.D. Translating the histone code. Science 293, 1074–1080 (2001).
Miller, R.A. & Ruddle, F.H. Pluripotent teratocarcinoma-thymus somatic cell hybrids. Cell 9, 45–55 (1976).
Blau, H.M., Chiu, C.P. & Webster, C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 32, 1171–1180 (1983).
Blau, H.M. et al. Plasticity of the differentiated state. Science 230, 758–766 (1985).
Tada, M., Takahama, Y., Abe, K., Nakatsuji, N. & Tada, T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 11, 1553–1558 (2001).
Cowan, C.A., Atienza, J., Melton, D.A. & Eggan, K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373 (2005).
Yamanaka, S. & Blau, H.M. Nuclear reprogramming to a pluripotent state by three approaches. Nature 465, 704–712 (2010).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Wernig, M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007).
Maherali, N. et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70 (2007).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Park, I.H. et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Hochedlinger, K. & Plath, K. Epigenetic reprogramming and induced pluripotency. Development 136, 509–523 (2009).
Jaenisch, R. & Young, R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132, 567–582 (2008).
Silva, J. & Smith, A. Capturing pluripotency. Cell 132, 532–536 (2008).
Davis, R.L., Weintraub, H. & Lassar, A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000 (1987).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Pang, Z.P. et al. Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223 (2011).
Huang, P. et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475, 386–389 (2011).
Hochedlinger, K. & Jaenisch, R. Nuclear reprogramming and pluripotency. Nature 441, 1061–1067 (2006).
Zhou, Q. & Melton, D.A. Extreme makeover: converting one cell into another. Cell Stem Cell 3, 382–388 (2008).
Graf, T. & Enver, T. Forcing cells to change lineages. Nature 462, 587–594 (2009).
Slack, J.M. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat. Rev. Mol. Cell Biol. 8, 369–378 (2007).
Zhou, Q., Brown, J., Kanarek, A., Rajagopal, J. & Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455, 627–632 (2008).
Feng, R. et al. PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc. Natl. Acad. Sci. USA 105, 6057–6062 (2008).
Xie, H., Ye, M., Feng, R. & Graf, T. Stepwise reprogramming of B cells into macrophages. Cell 117, 663–676 (2004).
Heinrich, C. et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 8, e1000373 (2010).
Heins, N. et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 5, 308–315 (2002).
Takeuchi, J.K. & Bruneau, B.G. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459, 708–711 (2009).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Szabo, E. et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468, 521–526 (2010).
Sekiya, S. & Suzuki, A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 475, 390–393 (2011).
Taylor, S.M. & Jones, P.A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell 17, 771–779 (1979).
Meissner, A. et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454, 766–770 (2008).
Taberlay, P.C. & Jones, P.A. DNA methylation and cancer. Prog. Drug Res. 67, 1–23 (2011).
De Carvalho, D.D., You, J.S. & Jones, P.A. DNA methylation and cellular reprogramming. Trends Cell Biol. 20, 609–617 (2010).
Chiu, C.P. & Blau, H.M. 5-Azacytidine permits gene activation in a previously noninducible cell type. Cell 40, 417–424 (1985).
Weintraub, H. et al. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc. Natl. Acad. Sci. USA 86, 5434–5438 (1989).
Choi, J. et al. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc. Natl. Acad. Sci. USA 87, 7988–7992 (1990).
Lassar, A.B., Paterson, B.M. & Weintraub, H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell 47, 649–656 (1986).
Weintraub, H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75, 1241–1244 (1993).
Schafer, B.W., Blakely, B.T., Darlington, G.J. & Blau, H.M. Effect of cell history on response to helix-loop-helix family of myogenic regulators. Nature 344, 454–458 (1990).
Wright, W.E. Induction of muscle genes in neural cells. J. Cell Biol. 98, 427–435 (1984).
Pavlath, G.K., Rich, K., Webster, S.G. & Blau, H.M. Localization of muscle gene products in nuclear domains. Nature 337, 570–573 (1989).
Weintraub, H. et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science 251, 761–766 (1991).
Blau, H.M. & Blakely, B.T. Plasticity of cell fate: insights from heterokaryons. Semin. Cell Dev. Biol. 10, 267–272 (1999).
Pomerantz, J.H., Mukherjee, S., Palermo, A.T. & Blau, H.M. Reprogramming to a muscle fate by fusion recapitulates differentiation. J. Cell Sci. 122, 1045–1053 (2009).
Buckingham, M. et al. The formation of skeletal muscle: from somite to limb. J. Anat. 202, 59–68 (2003).
Kassar-Duchossoy, L. et al. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature 431, 466–471 (2004).
Hasty, P. et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364, 501–506 (1993).
Nabeshima, Y. et al. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 364, 532–535 (1993).
Berkes, C.A. & Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 16, 585–595 (2005).
Tapscott, S.J. The circuitry of a master switch: MyoD and the regulation of skeletal muscle gene transcription. Development 132, 2685–2695 (2005).
Blais, A. et al. An initial blueprint for myogenic differentiation. Genes Dev. 19, 553–569 (2005).
Berkes, C.A. et al. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol. Cell 14, 465–477 (2004).
Albini, S. & Puri, P.L. SWI/SNF complexes, chromatin remodeling and skeletal myogenesis: it's time to exchange!. Exp. Cell Res. 316, 3073–3080 (2010).
Gerber, A.N., Klesert, T.R., Bergstrom, D.A. & Tapscott, S.J. Two domains of MyoD mediate transcriptional activation of genes in repressive chromatin: a mechanism for lineage determination in myogenesis. Genes Dev. 11, 436–450 (1997).
Cao, Y. et al. Genome-wide MyoD binding in skeletal muscle cells: a potential for broad cellular reprogramming. Dev. Cell 18, 662–674 (2010).
Molkentin, J.D., Black, B.L., Martin, J.F. & Olson, E.N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 83, 1125–1136 (1995).
Bergstrom, D.A. et al. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 9, 587–600 (2002).
de la Serna, I.L. et al. MyoD targets chromatin remodeling complexes to the myogenin locus prior to forming a stable DNA-bound complex. Mol. Cell Biol. 25, 3997–4009 (2005).
Puri, P.L. et al. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol. Cell 1, 35–45 (1997).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Kajimura, S., Seale, P. & Spiegelman, B.M. Transcriptional control of brown fat development. Cell Metab. 11, 257–262 (2010).
Kajimura, S. et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 460, 1154–1158 (2009).
Olson, E.N. Gene regulatory networks in the evolution and development of the heart. Science 313, 1922–1927 (2006).
Srivastava, D. Making or breaking the heart: from lineage determination to morphogenesis. Cell 126, 1037–1048 (2006).
Lickert, H. et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature 432, 107–112 (2004).
Cirillo, L.A. et al. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9, 279–289 (2002).
Smale, S.T. Pioneer factors in embryonic stem cells and differentiation. Curr. Opin. Genet. Dev. 20, 519–526 (2010).
Davidson, E.H. & Levine, M.S. Properties of developmental gene regulatory networks. Proc. Natl. Acad. Sci. USA 105, 20063–20066 (2008).
Reya, T., Morrison, S.J., Clarke, M.F. & Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Orkin, S.H. & Zon, L.I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 (2008).
Rovigatti, U. et al. Heavy chain immunoglobulin gene rearrangement in acute nonlymphocytic leukemia. Blood 63, 1023–1027 (1984).
Ha, K., Minden, M., Hozumi, N. & Gelfand, E.W. Immunoglobulin gene rearrangement in acute myelogenous leukemia. Cancer Res. 44, 4658–4660 (1984).
Palumbo, A., Minowada, J., Erikson, J., Croce, C.M. & Rovera, G. Lineage infidelity of a human myelogenous leukemia cell line. Blood 64, 1059–1063 (1984).
Tsai, S.F. et al. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature 339, 446–451 (1989).
Wall, L., deBoer, E. & Grosveld, F. The human beta-globin gene 3′ enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 2, 1089–1100 (1988).
Evans, T., Reitman, M. & Felsenfeld, G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc. Natl. Acad. Sci. USA 85, 5976–5980 (1988).
Pevny, L. et al. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349, 257–260 (1991).
Visvader, J.E., Elefanty, A.G., Strasser, A. & Adams, J.M. GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO J. 11, 4557–4564 (1992).
Kulessa, H., Frampton, J. & Graf, T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 9, 1250–1262 (1995).
Nerlov, C. & Graf, T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 12, 2403–2412 (1998).
Heyworth, C., Pearson, S., May, G. & Enver, T. Transcription factor-mediated lineage switching reveals plasticity in primary committed progenitor cells. EMBO J. 21, 3770–3781 (2002).
Kondo, M. et al. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature 407, 383–386 (2000).
Rolink, A.G., Nutt, S.L., Melchers, F. & Busslinger, M. Long-term in vivo reconstitution of T-cell development by Pax5-deficient B-cell progenitors. Nature 401, 603–606 (1999).
Mikkola, I., Heavey, B., Horcher, M. & Busslinger, M. Reversion of B cell commitment upon loss of Pax5 expression. Science 297, 110–113 (2002).
Cobaleda, C., Jochum, W. & Busslinger, M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature 449, 473–477 (2007).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Ghisletti, S. et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity 32, 317–328 (2010).
Natoli, G. Maintaining cell identity through global control of genomic organization. Immunity 33, 12–24 (2010).
Visel, A., Rubin, E.M. & Pennacchio, L.A. Genomic views of distant-acting enhancers. Nature 461, 199–205 (2009).
Visel, A. et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 457, 854–858 (2009).
Rada-Iglesias, A. et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283 (2011).
Creyghton, M.P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 107, 21931–21936 (2010).
Bussmann, L.H. et al. A robust and highly efficient immune cell reprogramming system. Cell Stem Cell 5, 554–566 (2009).
Hochedlinger, K., Yamada, Y., Beard, C. & Jaenisch, R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121, 465–477 (2005).
Lengner, C.J. et al. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell 1, 403–415 (2007).
Wang, V.E., Schmidt, T., Chen, J., Sharp, P.A. & Tantin, D. Embryonic lethality, decreased erythropoiesis, and defective octamer-dependent promoter activation in Oct-1-deficient mice. Mol. Cell. Biol. 24, 1022–1032 (2004).
Sebastiano, V. et al. Oct1 regulates trophoblast development during early mouse embryogenesis. Development 137, 3551–3560 (2010).
Corcoran, L.M. et al. Oct-2, although not required for early B-cell development, is critical for later B-cell maturation and for postnatal survival. Genes Dev. 7, 570–582 (1993).
Zhou, Q. et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev. Cell 13, 103–114 (2007).
Zaret, K.S. Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat. Rev. Genet. 9, 329–340 (2008).
Zaret, K.S. & Grompe, M. Generation and regeneration of cells of the liver and pancreas. Science 322, 1490–1494 (2008).
Murtaugh, L.C. & Melton, D.A. Genes, signals, and lineages in pancreas development. Annu. Rev. Cell Dev. Biol. 19, 71–89 (2003).
Bonal, C. & Herrera, P.L. Genes controlling pancreas ontogeny. Int. J. Dev. Biol. 52, 823–835 (2008).
Puri, S. & Hebrok, M. Cellular plasticity within the pancreas–lessons learned from development. Dev. Cell 18, 342–356 (2010).
Dor, Y., Brown, J., Martinez, O.I. & Melton, D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (2004).
Teta, M., Rankin, M.M., Long, S.Y., Stein, G.M. & Kushner, J.A. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell 12, 817–826 (2007).
Thorel, F. et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464, 1149–1154 (2010).
Zaret, K.S. & White, M.F. Diabetes forum: extreme makeover of pancreatic alpha-cells. Nature 464, 1132–1133 (2010).
Xu, X. et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 (2008).
Shen, C.N., Slack, J.M. & Tosh, D. Molecular basis of transdifferentiation of pancreas to liver. Nat. Cell Biol. 2, 879–887 (2000).
Ferber, S. et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 6, 568–572 (2000).
Kaneto, H. et al. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes 54, 1009–1022 (2005).
Miyatsuka, T. et al. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem. Biophys. Res. Commun. 310, 1017–1025 (2003).
Sapir, T. et al. Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc. Natl. Acad. Sci. USA 102, 7964–7969 (2005).
Horb, M.E., Shen, C.N., Tosh, D. & Slack, J.M. Experimental conversion of liver to pancreas. Curr. Biol. 13, 105–115 (2003).
Grapin-Botton, A., Majithia, A.R. & Melton, D.A. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 15, 444–454 (2001).
Gu, G., Dubauskaite, J. & Melton, D.A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002).
Jonsson, J., Carlsson, L., Edlund, T. & Edlund, H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371, 606–609 (1994).
Ahlgren, U., Jonsson, J., Jonsson, L., Simu, K. & Edlund, H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 12, 1763–1768 (1998).
Waeber, G., Thompson, N., Nicod, P. & Bonny, C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol. Endocrinol. 10, 1327–1334 (1996).
Watada, H. et al. The human glucokinase gene beta-cell-type promoter: an essential role of insulin promoter factor 1/PDX-1 in its activation in HIT-T15 cells. Diabetes 45, 1478–1488 (1996).
Apelqvist, A. et al. Notch signalling controls pancreatic cell differentiation. Nature 400, 877–881 (1999).
Yechoor, V. et al. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev. Cell 16, 358–373 (2009).
Desgraz, R. & Herrera, P.L. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development 136, 3567–3574 (2009).
Gradwohl, G., Dierich, A., LeMeur, M. & Guillemot, F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 97, 1607–1611 (2000).
Wang, S. et al. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc. Natl. Acad. Sci. USA 106, 9715–9720 (2009).
Gasa, R. et al. Induction of pancreatic islet cell differentiation by the neurogenin-neuroD cascade. Differentiation 76, 381–391 (2008).
Gasa, R. et al. Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. Proc. Natl. Acad. Sci. USA 101, 13245–13250 (2004).
Gu, C. et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 11, 298–310 (2010).
Kojima, H. et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat. Med. 9, 596–603 (2003).
Bertrand, N., Castro, D.S. & Guillemot, F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517–530 (2002).
Olbrot, M., Rud, J., Moss, L.G. & Sharma, A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc. Natl. Acad. Sci. USA 99, 6737–6742 (2002).
Kataoka, K. et al. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J. Biol. Chem. 277, 49903–49910 (2002).
Matsuoka, T.A. et al. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol. Cell Biol. 23, 6049–6062 (2003).
Artner, I., Hang, Y., Guo, M., Gu, G. & Stein, R. MafA is a dedicated activator of the insulin gene in vivo. J. Endocrinol. 198, 271–279 (2008).
Zhang, C. et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell Biol. 25, 4969–4976 (2005).
Nishimura, W., Bonner-Weir, S. & Sharma, A. Expression of MafA in pancreatic progenitors is detrimental for pancreatic development. Dev. Biol. 333, 108–120 (2009).
Matsuoka, T.A. et al. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc. Natl. Acad. Sci. USA 101, 2930–2933 (2004).
Nishimura, W. et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev. Biol. 293, 526–539 (2006).
Kaneto, H. et al. A crucial role of MafA as a novel therapeutic target for diabetes. J. Biol. Chem. 280, 15047–15052 (2005).
Wilson, S.W. & Houart, C. Early steps in the development of the forebrain. Dev. Cell 6, 167–181 (2004).
Caspary, T. & Anderson, K.V. Patterning cell types in the dorsal spinal cord: what the mouse mutants say. Nat. Rev. Neurosci. 4, 289–297 (2003).
Jessell, T.M. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 1, 20–29 (2000).
Guillemot, F. Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr. Opin. Cell Biol. 17, 639–647 (2005).
Molyneaux, B.J., Arlotta, P., Menezes, J.R. & Macklis, J.D. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 8, 427–437 (2007).
Wonders, C.P. & Anderson, S.A. The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 7, 687–696 (2006).
Rowitch, D.H. Glial specification in the vertebrate neural tube. Nat. Rev. Neurosci. 5, 409–419 (2004).
Jan, Y.N. & Jan, L.Y. Neuronal cell fate specification in Drosophila. Curr. Opin. Neurobiol. 4, 8–13 (1994).
Ross, S.E., Greenberg, M.E. & Stiles, C.D. Basic helix-loop-helix factors in cortical development. Neuron 39, 13–25 (2003).
Lee, J.E. et al. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 268, 836–844 (1995).
Turner, D.L. & Weintraub, H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 8, 1434–1447 (1994).
Bellefroid, E.J. et al. X-MyT1, a Xenopus C2HC-type zinc finger protein with a regulatory function in neuronal differentiation. Cell 87, 1191–1202 (1996).
Perez, S.E., Rebelo, S. & Anderson, D.J. Early specification of sensory neuron fate revealed by expression and function of neurogenins in the chick embryo. Development 126, 1715–1728 (1999).
Ma, Q., Kintner, C. & Anderson, D.J. Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87, 43–52 (1996).
Farah, M.H. et al. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 127, 693–702 (2000).
Nieto, M., Schuurmans, C., Britz, O. & Guillemot, F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron 29, 401–413 (2001).
Lo, L., Sommer, L. & Anderson, D.J. MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells. Curr. Biol. 7, 440–450 (1997).
Louvi, A. & Artavanis-Tsakonas, S. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93–102 (2006).
Casarosa, S., Fode, C. & Guillemot, F. Mash1 regulates neurogenesis in the ventral telencephalon. Development 126, 525–534 (1999).
Fode, C. et al. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 14, 67–80 (2000).
Parras, C.M. et al. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 16, 324–338 (2002).
Sommer, L., Shah, N., Rao, M. & Anderson, D.J. The cellular function of MASH1 in autonomic neurogenesis. Neuron 15, 1245–1258 (1995).
Guillemot, F. Spatial and temporal specification of neural fates by transcription factor codes. Development 134, 3771–3780 (2007).
Shirasaki, R. & Pfaff, S.L. Transcriptional codes and the control of neuronal identity. Annu. Rev. Neurosci. 25, 251–281 (2002).
Guillemot, F. et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75, 463–476 (1993).
Kondo, T. & Raff, M. Basic helix-loop-helix proteins and the timing of oligodendrocyte differentiation. Development 127, 2989–2998 (2000).
Sugimori, M. et al. Ascl1 is required for oligodendrocyte development in the spinal cord. Development 135, 1271–1281 (2008).
Parras, C.M. et al. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J. Neurosci. 27, 4233–4242 (2007).
Petryniak, M.A., Potter, G.B., Rowitch, D.H. & Rubenstein, J.L. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron 55, 417–433 (2007).
Jessberger, S., Toni, N., Clemenson, G.D. Jr., Ray, J. & Gage, F.H. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat. Neurosci. 11, 888–893 (2008).
Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184 (2009).
Kondo, T. & Raff, M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289, 1754–1757 (2000).
Malatesta, P., Hartfuss, E. & Gotz, M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127, 5253–5263 (2000).
Noctor, S.C., Flint, A.C., Weissman, T.A., Dammerman, R.S. & Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 (2001).
Berninger, B. et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 27, 8654–8664 (2007).
Mori, T. et al. Inducible gene deletion in astroglia and radial glia–a valuable tool for functional and lineage analysis. Glia 54, 21–34 (2006).
Blum, R. et al. Neuronal network formation from reprogrammed early postnatal rat cortical glial cells. Cereb. Cortex 21, 413–424 (2011).
Gotz, M. Making glutamatergic neurons from GABAergic progenitors. Nat. Neurosci. 13, 1308–1309 (2010).
Arber, S. et al. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23, 659–674 (1999).
Rouaux, C. & Arlotta, P. Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nat. Neurosci. 13, 1345–1347 (2010).
Cheng, L. et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat. Neurosci. 7, 510–517 (2004).
Leone, D.P., Srinivasan, K., Chen, B., Alcamo, E. & McConnell, S.K. The determination of projection neuron identity in the developing cerebral cortex. Curr. Opin. Neurobiol. 18, 28–35 (2008).
McEvilly, R.J., de Diaz, M.O., Schonemann, M.D., Hooshmand, F. & Rosenfeld, M.G. Transcriptional regulation of cortical neuron migration by POU domain factors. Science 295, 1528–1532 (2002).
Sugitani, Y. et al. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 16, 1760–1765 (2002).
Pfisterer, U. et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. USA 108, 10343–10348 (2011).
Yoo, A.S. et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476, 228–231 (2011).
Caiazzo, M. et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 (2011).
Wang, S. et al. Myt1 and Ngn3 form a feed-forward expression loop to promote endocrine islet cell differentiation. Dev. Biol. 317, 531–540 (2008).
Weiner, J.A. & Chun, J. Png-1, a nervous system-specific zinc finger gene, identifies regions containing postmitotic neurons during mammalian embryonic development. J. Comp. Neurol. 381, 130–142 (1997).
Cahoy, J.D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Armstrong, R.C., Kim, J.G. & Hudson, L.D. Expression of myelin transcription factor I (MyTI), a “zinc-finger” DNA-binding protein, in developing oligodendrocytes. Glia 14, 303–321 (1995).
Gu, G. et al. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development 131, 165–179 (2004).
Castro, D.S. et al. Proneural bHLH and Brn proteins coregulate a neurogenic program through cooperative binding to a conserved DNA motif. Dev. Cell 11, 831–844 (2006).
Witta, S.E., Agarwal, V.R. & Sato, S.M. XIPOU 2, a noggin-inducible gene, has direct neuralizing activity. Development 121, 721–730 (1995).
Huangfu, D. et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 26, 795–797 (2008).
Tursun, B., Patel, T., Kratsios, P. & Hobert, O. Direct conversion of C. elegans germ cells into specific neuron types. Science 331, 304–308 (2011).
Singhal, N. et al. Chromatin-remodeling components of the BAF complex facilitate reprogramming. Cell 141, 943–955 (2010).
Anokye-Danso, F. et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 8, 376–388 (2011).
Miyoshi, N. et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell 8, 633–638 (2011).
Goldsmith, M.A., Desai, D.M., Schultz, T. & Weiss, A. Function of a heterologous muscarinic receptor in T cell antigen receptor signal transduction mutants. J. Biol. Chem. 264, 17190–17197 (1989).
Fambrough, D., McClure, K., Kazlauskas, A. & Lander, E.S. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell 97, 727–741 (1999).
Davidson, E.H. Emerging properties of animal gene regulatory networks. Nature 468, 911–920 (2010).
Probst, A.V., Dunleavy, E. & Almouzni, G. Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol. 10, 192–206 (2009).
Hanna, J. et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595–601 (2009).
Hong, H. et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 460, 1132–1135 (2009).
Utikal, J. et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460, 1145–1148 (2009).
Marion, R.M. et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460, 1149–1153 (2009).
Li, H. et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 460, 1136–1139 (2009).
Kawamura, T. et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460, 1140–1144 (2009).
Bhutani, N. et al. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature 463, 1042–1047 (2010).
Meissner, A., Wernig, M. & Jaenisch, R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat. Biotechnol. 25, 1177–1181 (2007).
Wernig, M. et al. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat. Biotechnol. 26, 916–924 (2008).
Ptashne, M. & Gann, A. Genes & Signals (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2002).
Davidson, E.H. The Regulatory Genome: Gene Regulatory Networks in Development and Evolution (Academic, Burlington, MA; San Diego, 2006).
Li, B., Carey, M. & Workman, J.L. The role of chromatin during transcription. Cell 128, 707–719 (2007).
Ho, L. & Crabtree, G.R. Chromatin remodelling during development. Nature 463, 474–484 (2010).
Suzuki, M.M. & Bird, A. DNA methylation landscapes: provocative insights from epigenomics. Nat. Rev. Genet. 9, 465–476 (2008).
Ma, D.K. et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077 (2009).
Zaret, K.S. et al. Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm. Cold Spring Harb. Symp. Quant. Biol. 73, 119–126 (2008).
Niwa, H. How is pluripotency determined and maintained? Development 134, 635–646 (2007).
Erwin, D.H. & Davidson, E.H. The evolution of hierarchical gene regulatory networks. Nat. Rev. Genet. 10, 141–148 (2009).
Stadtfeld, M., Maherali, N., Breault, D.T. & Hochedlinger, K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2, 230–240 (2008).
Mikkelsen, T.S. et al. Dissecting direct reprogramming through integrative genomic analysis. Nature 454, 49–55 (2008).
Aoi, T. et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321, 699–702 (2008).
Hanna, J. et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 133, 250–264 (2008).
Sun, Y. et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104, 365–376 (2001).
Frankel, N. et al. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466, 490–493 (2010).
Ng, R.K. & Gurdon, J.B. Epigenetic memory of active gene transcription is inherited through somatic cell nuclear transfer. Proc. Natl. Acad. Sci. USA 102, 1957–1962 (2005).
Biddle, A., Simeoni, I. & Gurdon, J.B. Xenopus oocytes reactivate muscle gene transcription in transplanted somatic nuclei independently of myogenic factors. Development 136, 2695–2703 (2009).
Polo, J.M. et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol. 28, 848–855 (2010).
Kim, K. et al. Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 (2010).
Gaspard, N., Gaillard, A. & Vanderhaeghen, P. Making cortex in a dish: in vitro corticopoiesis from embryonic stem cells. Cell Cycle 8, 2491–2496 (2009).
Saha, K. & Jaenisch, R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell 5, 584–595 (2009).
Stadtfeld, M. & Hochedlinger, K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 24, 2239–2263 (2010).
Lin, W. et al. Foxa1 and Foxa2 function both upstream of and cooperatively with Lmx1a and Lmx1b in a feedforward loop promoting mesodiencephalic dopaminergic neuron development. Dev. Biol. 333, 386–396 (2009).
Omodei, D. et al. Anterior-posterior graded response to Otx2 controls proliferation and differentiation of dopaminergic progenitors in the ventral mesencephalon. Development 135, 3459–3470 (2008).
Andersson, E. et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell 124, 393–405 (2006).
Nishiyama, A. et al. Uncovering early response of gene regulatory networks in ESCs by systematic induction of transcription factors. Cell Stem Cell 5, 420–433 (2009).
Hobert, O., Carrera, I. & Stefanakis, N. The molecular and gene regulatory signature of a neuron. Trends Neurosci. 33, 435–445 (2010).
Gaspard, N. & Vanderhaeghen, P. Mechanisms of neural specification from embryonic stem cells. Curr. Opin. Neurobiol. 20, 37–43 (2010).
Gaspard, N. et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455, 351–357 (2008).
Zhang, X. et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 7, 90–100 (2010).
Kriegstein, A., Noctor, S. & Martinez-Cerdeno, V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 7, 883–890 (2006).
Murry, C.E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
Elkabetz, Y. et al. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 22, 152–165 (2008).
Chambers, S.M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Koch, P., Opitz, T., Steinbeck, J.A., Ladewig, J. & Brustle, O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. USA 106, 3225–3230 (2009).
Hu, B.Y. et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 107, 4335–4340 (2010).
Lee, G. et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406 (2009).
Marchetto, M.C. et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 (2010).
Brennand, K.J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011).
Nguyen, H.N. et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8, 267–280 (2011).
Carvajal-Vergara, X. et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 465, 808–812 (2010).
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011).
Angel, M. & Yanik, M.F. Innate immune suppression enables frequent transfection with RNA encoding reprogramming proteins. PLoS ONE 5, e11756 (2010).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 (2010).
Laiosa, C.V. et al. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity 25, 731–744 (2006).
Acknowledgements
We would like to thank the many colleagues who have contributed to this work through many insightful discussions on the topic over the last year. These include T. Graf, F. Guillemot, M. Götz, J. Crabtree, A. Smith, R. Jaenisch, J. Wysocka, H. Blau, H. Chang, A. Kriegstein and I. Weissman. M.W. is a New York Stem Cell Foundation (NYSCF) Robertson Investigator, and our research is also supported by grants from the US National Institutes of Health, the Department of Defense, the California Institute of Regenerative Medicine, the BioX program at Stanford, the Baxter Foundation and the Stinehart-Reed Foundation. T.V. is supported by the Ruth and Robert Halperin Stanford Graduate Fellowship. We apologize to numerous authors whose valuable contributions we were unable to cite due to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Vierbuchen, T., Wernig, M. Direct lineage conversions: unnatural but useful?. Nat Biotechnol 29, 892–907 (2011). https://doi.org/10.1038/nbt.1946
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1946
This article is cited by
-
Pro-neuronal activity of Myod1 due to promiscuous binding to neuronal genes
Nature Cell Biology (2020)
-
Early Intervention in Ischemic Tissue with Oxygen Nanocarriers Enables Successful Implementation of Restorative Cell Therapies
Cellular and Molecular Bioengineering (2020)
-
Direct conversion of human fibroblasts into therapeutically active vascular wall-typical mesenchymal stem cells
Cellular and Molecular Life Sciences (2020)
-
Cell identity reprogrammed
Nature (2019)
-
Alterations in Beta Cell Identity in Type 1 and Type 2 Diabetes
Current Diabetes Reports (2019)