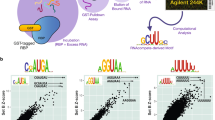
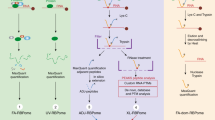
Abstract
Metazoan genomes encode hundreds of RNA-binding proteins (RBPs) but RNA-binding preferences for relatively few RBPs have been well defined1. Current techniques for determining RNA targets, including in vitro selection and RNA co-immunoprecipitation2,3,4,5, require significant time and labor investment. Here we introduce RNAcompete, a method for the systematic analysis of RNA binding specificities that uses a single binding reaction to determine the relative preferences of RBPs for short RNAs that contain a complete range of k-mers in structured and unstructured RNA contexts. We tested RNAcompete by analyzing nine diverse RBPs (HuR, Vts1, FUSIP1, PTB, U1A, SF2/ASF, SLM2, RBM4 and YB1). RNAcompete identified expected and previously unknown RNA binding preferences. Using in vitro and in vivo binding data, we demonstrate that preferences for individual 7-mers identified by RNAcompete are a more accurate representation of binding activity than are conventional motif models. We anticipate that RNAcompete will be a valuable tool for the study of RNA-protein interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Glisovic, T., Bachorik, J.L., Yong, J. & Dreyfuss, G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 582, 1977–1986 (2008).
Tenenbaum, S.A., Carson, C.C., Lager, P.J. & Keene, J.D. Identifying mRNA subsets in messenger ribonucleoprotein complexes by using cDNA arrays. Proc. Natl. Acad. Sci. USA 97, 14085–14090 (2000).
Gerber, A.P., Herschlag, D. & Brown, P.O. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol. 2, E79 (2004).
Ule, J., Jensen, K., Mele, A. & Darnell, R.B. CLIP: a method for identifying protein-RNA interaction sites in living cells. Methods 37, 376–386 (2005).
Tuerk, C. & Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990).
Auweter, S.D., Oberstrass, F.C. & Allain, F.H. Sequence-specific binding of single-stranded RNA: is there a code for recognition? Nucleic Acids Res. 34, 4943–4959 (2006).
Berger, M.F. et al. Compact, universal DNA microarrays to comprehensively determine transcription-factor binding site specificities. Nat. Biotechnol. 24, 1429–1435 (2006).
Philippakis, A.A., Qureshi, A.M., Berger, M.F. & Bulyk, M.L. Design of compact, universal DNA microarrays for protein binding microarray experiments. J. Comput. Biol. 15, 655–665 (2008).
Myer, V.E., Fan, X.C. & Steitz, J.A. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. EMBO J. 16, 2130–2139 (1997).
Levine, T.D., Gao, F., King, P.H., Andrews, L.G. & Keene, J.D. Hel-N1: an autoimmune RNA-binding protein with specificity for 3′ uridylate-rich untranslated regions of growth factor mRNAs. Mol. Cell. Biol. 13, 3494–3504 (1993).
Aviv, T., Lin, Z., Ben-Ari, G., Smibert, C.A. & Sicheri, F. Sequence-specific recognition of RNA hairpins by the SAM domain of Vts1p. Nat. Struct. Mol. Biol. 13, 168–176 (2006).
Sengupta, S. et al. The RNA-binding protein HuR regulates the expression of cyclooxygenase-2. J. Biol. Chem. 278, 25227–25233 (2003).
Meisner, N.C. et al. mRNA openers and closers: modulating AU-rich element-controlled mRNA stability by a molecular switch in mRNA secondary structure. ChemBioChem 5, 1432–1447 (2004).
Tsai, D.E., Harper, D.S. & Keene, J.D. U1-snRNP-A protein selects a ten nucleotide consensus sequence from a degenerate RNA pool presented in various structural contexts. Nucleic Acids Res. 19, 4931–4936 (1991).
Tacke, R. & Manley, J.L. The human splicing factors ASF/SF2 and SC35 possess distinct, functionally significant RNA binding specificities. EMBO J. 14, 3540–3551 (1995).
Perez, I., McAfee, J.G. & Patton, J.G. Multiple RRMs contribute to RNA binding specificity and affinity for polypyrimidine tract binding protein. Biochemistry 36, 11881–11890 (1997).
Gao, F.B., Carson, C.C., Levine, T. & Keene, J.D. Selection of a subset of mRNAs from combinatorial 3′ untranslated region libraries using neuronal RNA-binding protein Hel-N1. Proc. Natl. Acad. Sci. USA 91, 11207–11211 (1994).
Aviv, T. et al. The RNA-binding SAM domain of Smaug defines a new family of post-transcriptional regulators. Nat. Struct. Biol. 10, 614–621 (2003).
Shin, C. & Manley, J.L. The SR protein SRp38 represses splicing in M phase cells. Cell 111, 407–417 (2002).
Lai, M.C., Kuo, H.W., Chang, W.C. & Tarn, W.Y. A novel splicing regulator shares a nuclear import pathway with SR proteins. EMBO J. 22, 1359–1369 (2003).
Lin, Q., Taylor, S.J. & Shalloway, D. Specificity and determinants of Sam68 RNA binding. Implications for the biological function of K homology domains. J. Biol. Chem. 272, 27274–27280 (1997).
Ohno, G., Hagiwara, M. & Kuroyanagi, H. STAR family RNA-binding protein ASD-2 regulates developmental switching of mutually exclusive alternative splicing in vivo. Genes Dev. 22, 360–374 (2008).
Stickeler, E. et al. The RNA binding protein YB-1 binds A/C-rich exon enhancers and stimulates splicing of the CD44 alternative exon v4. EMBO J. 20, 3821–3830 (2001).
Dong, J. et al. RNA-binding specificity of Y-box protein 1. RNA Biol. 6, 59–64 (2009).
Skabkina, O.V., Lyabin, D.N., Skabkin, M.A. & Ovchinnikov, L.P. YB-1 autoregulates translation of its own mRNA at or prior to the step of 40S ribosomal subunit joining. Mol. Cell. Biol. 25, 3317–3323 (2005).
Chen, X., Hughes, T.R. & Morris, Q. RankMotif.: a motif-search algorithm that accounts for relative ranks of K-mers in binding transcription factors. Bioinformatics 23, i72–i79 (2007).
Sanford, J.R. Identification of nuclear and cytoplasmic mRNA targets for the shuttling protein SF2/ASF. PLoS One 3, e3369 (2008).
Sanford, J.R. et al. Splicing factor SFRS1 recognizes a functionally diverse landscape of RNA transcripts. Genome Res. 19, 381–394 (2009).
Oberstrass, F.C. et al. Structure of PTB bound to RNA: specific binding and implications for splicing regulation. Science 309, 2054–2057 (2005).
Liu, H.X., Zhang, M. & Krainer, A.R. Identification of functional exonic splicing enhancer motifs recognized by individual SR proteins. Genes Dev. 12, 1998–2012 (1998).
Gama-Carvalho, M., Barbosa-Morais, N.L., Brodsky, A.S., Silver, P.A. & Carmo-Fonseca, M. Genome-wide identification of functionally distinct subsets of cellular mRNAs associated with two nucleocytoplasmic-shuttling mammalian splicing factors. Genome Biol. 7, R113 (2006).
Steffen, P., Voss, B., Rehmsmeier, M., Reeder, J. & Giegerich, R. RNAshapes: an integrated RNA analysis package based on abstract shapes. Bioinformatics 22, 500–503 (2006).
Hofacker, I.L. et al. Fast folding and comparison of RNA secondary structures. Monatsh. Chem. 125, 167–188 (1994).
Huber, W., von Heydebreck, A., Sultmann, H., Poustka, A. & Vingron, M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18 Suppl 1, S96–S104 (2002).
Hughes, J.D., Estep, P.W., Tavazoie, S. & Church, G.M. Computational identification of cis-regulatory elements associated with groups of functionally related genes in Saccharomyces cerevisiae. J. Mol. Biol. 296, 1205–1214 (2000).
Bailey, T.L., Williams, N., Misleh, C. & Li, W.W. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 34, W369–373 (2006).
Hiller, M., Pudimat, R., Busch, A. & Backofen, R. Using RNA secondary structures to guide sequence motif finding towards single-stranded regions. Nucleic Acids Res. 34, e117 (2006).
Frey, B.J. & Dueck, D. Clustering by passing messages between data points. Science 315, 972–976 (2007).
Acknowledgements
We are grateful to C. Smibert, H. Lipshitz, F. Sicheri, M. Kekis and T. Babak for helpful commentary. Bacterial expression plasmids for the N-terminal arm of U1A, the Vts1 SAM domain, full-length PTB and YB1 were generously provided by C. Lutz (Univ. of Medicine and Dentistry of New Jersey), C. Smibert (Univ. of Toronto), M. Garcia-Blanco (Duke Univ.) and K. Kohno (Univ. of Occupational and Environmental Health, Kitakyushu, Japan). This work was supported by grants to T.R.H., B.J.B. and Q.M. from CIHR (MOP-49451, MOP-14609, MOP-93671), by Natural Sciences and Engineering Research Council operating and Canadian Foundation of Innovation grants to Q.M., Genome Canada through the Ontario Genomics Institute and the Ontario Research Fund. D.R. was supported in part by a National Science and Engineering Research Council of Canada (NSERC) postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
D.R. developed the method and performed the experiments; D.R., H.K., Q.M. and T.R.H. designed the array, processed and analyzed the data, wrote the paper and made the figures; E.C. and L.P.C. contributed to the motif analyses and cross-validation; S.C. and S.T. assisted with cloning and protein production; B.J.B., Q.M. and T.R.H. conceived of the method and supported the project.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Table 1 (PDF 394 kb)
Rights and permissions
About this article
Cite this article
Ray, D., Kazan, H., Chan, E. et al. Rapid and systematic analysis of the RNA recognition specificities of RNA-binding proteins. Nat Biotechnol 27, 667–670 (2009). https://doi.org/10.1038/nbt.1550
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1550
This article is cited by
-
Improved modeling of RNA-binding protein motifs in an interpretable neural model of RNA splicing
Genome Biology (2024)
-
Dynamic changes in LINC00458/HBL1 lncRNA expression during hiPSC differentiation to cardiomyocytes
Scientific Reports (2024)
-
Towards in silico CLIP-seq: predicting protein-RNA interaction via sequence-to-signal learning
Genome Biology (2023)
-
YBX1/lncRNA SBF2-AS1 interaction regulates proliferation and tamoxifen sensitivity via PI3K/AKT/MTOR signaling in breast cancer cells
Molecular Biology Reports (2023)
-
Simple synthesis of massively parallel RNA microarrays via enzymatic conversion from DNA microarrays
Nature Communications (2022)