Abstract
The fusion pore is the first crucial intermediate formed during exocytosis, yet little is known about the mechanisms that determine the size and kinetic properties of these transient structures1. Here, we reduced the number of available SNAREs (proteins that mediate vesicle fusion) in neurons and observed changes in transmitter release that are suggestive of alterations in fusion pores. To investigate these changes, we employed reconstituted fusion assays using nanodiscs to trap pores in their initial open state. Optical measurements revealed that increasing the number of SNARE complexes enhanced the rate of release from single pores and enabled the escape of larger cargoes. To determine whether this effect was due to changes in nascent pore size or to changes in stability, we developed an approach that uses nanodiscs and planar lipid bilayer electrophysiology to afford microsecond resolution at the single event level. Both pore size and stability were affected by SNARE copy number. Increasing the number of vesicle (v)-SNAREs per nanodisc from three to five caused a twofold increase in pore size and decreased the rate of pore closure by more than three orders of magnitude. Moreover, pairing of v-SNAREs and target (t)-SNAREs to form trans-SNARE complexes was highly dynamic: flickering nascent pores closed upon addition of a v-SNARE fragment, revealing that the fully assembled, stable SNARE complex does not form at this stage of exocytosis. Finally, a deletion at the base of the SNARE complex, which mimics the action of botulinum neurotoxin A, markedly reduced fusion pore stability. In summary, trans-SNARE complexes are dynamic, and the number of SNAREs recruited to drive fusion determines fundamental properties of individual pores.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Giraudo, C. G. et al. SNAREs can promote complete fusion and hemifusion as alternative outcomes. J. Cell Biol. 170, 249–260 (2005)
Fulop, T., Radabaugh, S. & Smith, C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J. Neurosci. 25, 7324–7332 (2005)
Richards, D. A. Vesicular release mode shapes the postsynaptic response at hippocampal synapses. J. Physiol. (Lond.) 587, 5073–5080 (2009)
Choi, S., Klingauf, J. & Tsien, R. W. Fusion pore modulation as a presynaptic mechanism contributing to expression of long-term potentiation. Phil. Trans. R. Soc. Lond. B 358, 695–705 (2003)
Alabi, A. A. & Tsien, R. W. Perspectives on kiss-and-run: role in exocytosis, endocytosis, and neurotransmission. Annu. Rev. Physiol. 75, 393–422 (2013)
Renger, J. J., Egles, C. & Liu, G. A developmental switch in neurotransmitter flux enhances synaptic efficacy by affecting AMPA receptor activation. Neuron 29, 469–484 (2001)
Shi, L. et al. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science 335, 1355–1359 (2012)
Liu, G., Choi, S. & Tsien, R. W. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron 22, 395–409 (1999)
Bao, H. et al. Exocytotic fusion pores are composed of both lipids and proteins. Nat. Struct. Mol. Biol. 23, 67–73 (2016)
Mueller, P., Rudin, D. O., Tien, H. T. & Wescott, W. C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194, 979–980 (1962)
Wu, Z. et al. Nanodisc–cell fusion: control of fusion pore nucleation and lifetimes by SNARE protein transmembrane domains. Sci. Rep. 6, 27287 (2016)
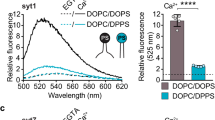
Bhalla, A., Chicka, M. C., Tucker, W. C. & Chapman, E. R. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 13, 323–330 (2006)
Wiederhold, K. et al. A coiled coil trigger site is essential for rapid binding of synaptobrevin to the SNARE acceptor complex. J. Biol. Chem. 285, 21549–21559 (2010)
Südhof, T. C. & Rothman, J. E. Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477 (2009)
Albillos, A. et al. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature 389, 509–512 (1997)
Klyachko, V. A. & Jackson, M. B. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature 418, 89–92 (2002)
Henkel, A. W., Meiri, H., Horstmann, H., Lindau, M. & Almers, W. Rhythmic opening and closing of vesicles during constitutive exo- and endocytosis in chromaffin cells. EMBO J. 19, 84–93 (2000)
Staal, R. G. W., Mosharov, E. V. & Sulzer, D. Dopamine neurons release transmitter via a flickering fusion pore. Nat. Neurosci. 7, 341–346 (2004)
Wu, Z. Y. et al. Dilation of fusion pores by crowding of SNARE proteins. Elife 6, e22964 (2017)
Sinha, R., Ahmed, S., Jahn, R. & Klingauf, J. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc. Natl Acad. Sci. USA 108, 14318–14323 (2011)
Lai, Y. et al. Fusion pore formation and expansion induced by Ca2+ and synaptotagmin 1. Proc. Natl Acad. Sci. USA 110, 1333–1338 (2013)
Wickner, W. & Schekman, R. Membrane fusion. Nat. Struct. Mol. Biol. 15, 658–664 (2008)
Orso, G. et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature 460, 978–983 (2009)
Cao, Y. L. et al. MFN1 structures reveal nucleotide-triggered dimerization critical for mitochondrial fusion. Nature 542, 372–376 (2017)
Podbilewicz, B. Virus and cell fusion mechanisms. Annu. Rev. Cell Dev. Biol. 30, 111–139 (2014)
Yeh, F. L. et al. SV2 mediates entry of tetanus neurotoxin into central neurons. PLOS Pathogens 6, e1001207 (2010)
Kutner, R. H., Zhang, X. Y. & Reiser, J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protocols 4, 495–505 (2009)
Nasr, M. L. et al. Covalently circularized nanodiscs for studying membrane proteins and viral entry. Nat. Methods 14, 49–52 (2017)
Marvin, J. S., Schreiter, E. R., Echevarría, I. M. & Looger, L. L. A genetically encoded, high-signal-to-noise maltose sensor. Proteins 79, 3025–3036 (2011)
Tucker, W. C., Weber, T. & Chapman, E. R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004)
Bao, H. & Duong, F. Discovery of an auto-regulation mechanism for the maltose ABC transporter MalFGK2. PLoS ONE 7, e34836 (2012)
Brooks, B. R. et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009)
Eastman, P. et al. OpenMM 4: a reusable, extensible, hardware independent library for high performance molecular simulation. J. Chem. Theory Comput. 9, 461–469 (2013)
Guvench, O. et al. Additive empirical force field for hexopyranose monosaccharides. J. Comput. Chem. 29, 2543–2564 (2008)
Guvench, O., Hatcher, E. R., Venable, R. M., Pastor, R. W. & Mackerell, A. D. CHARMM additive all-atom force field for glycosidic linkages between hexopyranoses. J. Chem. Theory Comput. 5, 2353–2370 (2009)
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983)
Bohne, A., Lang, E. & von der Lieth, C. W. SWEET—WWW-based rapid 3D construction of oligo- and polysaccharides. Bioinformatics 15, 767–768 (1999)
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008)
Jo, S., Song, K. C., Desaire, H., MacKerell, A. D. Jr & Im, W. Glycan Reader: automated sugar identification and simulation preparation for carbohydrates and glycoproteins. J. Comput. Chem. 32, 3135–3141 (2011)
Kyoung, M., Zhang, Y., Diao, J., Chu, S. & Brunger, A. T. Studying calcium-triggered vesicle fusion in a single vesicle-vesicle content and lipid-mixing system. Nat. Protocols 8, 1–16 (2013)
Finkelstein, A. Bilayers: formation, measurements, and incorporation of components. Methods Enzymol. 32, 489–501 (1974)
Hille, B. Ion Channels of Excitable Membranes 3rd edn (Sinauer, 2001)
Acknowledgements
This study was supported by grants from the NIH (MH061876 and NS097362 to E.R.C.; NS081293 to B.C.). H.B. was supported by a postdoctoral fellowship from the Human Frontier Science Program. D.R. was supported by an NIH fellowship (F32GM112371). E.R.C. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
H.B. and E.R.C. conceived of the project and designed the biochemistry experiments. H.B. performed nanodisc reconstitution and fusion assays. H.B. and D.D. performed the planar lipid bilayer recordings. N.A.C. designed and conducted the experiments using neurons. Y.J. and B.C. aided in the initial planar lipid bilayer recordings. J.S.B. contributed neurons. X.L. and H.B. contributed to the single vesicle fusion assays. D.R. and Q.C. conducted molecular dynamics simulations. H.B., D.D., N.A.C. and E.R.C. wrote the paper, and all other authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks J. Dittman, R. Heidelberger, J. Sørensen and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
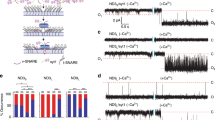
Extended Data Figure 1 Viral expression of cd-SYB2.
a, cDNA encoding the cytosolic domain of SYB2 (cd-SYB2, residues 1–95) was cloned into a FUGW transfer vector modified to have a synapsin promoter and to co-express soluble eGFP via an IRES sequence; eGFP serves as a marker for infection efficiency. For control experiments, eGFP alone was expressed. Both constructs were packaged into lentivirus for expression in neuronal cultures. b, Representative images of cells stained for a neuronal marker (MAP2, magenta) and GFP (green). Images were adjusted for brightness and contrast for the sake of presentation. Both preparations used for Fig. 1g were examined and had similar GFP expression levels and coverage across cells. The scale bar (50 μm) applies to all nine images shown. c, Quantification of the ICC demonstrating that both cd-SYB2 and control viruses achieved a nearly 100% infection rate. Per cent infected refers to the number of visually identified MAP2-positive somas (that is, neurons) that were also positive for GFP. Three fields of view were quantified for each condition. d, Representative traces (left) and quantification (right) demonstrating that cd-SYB2 was expressed at levels sufficient to inhibit evoked IPSCs triggered by field stimulation (P = 0.032, two-tailed t-test; n = 10 neurons for each condition, using two litters of mice, three coverslips per condition). Data are presented as mean ± s.e.m. *P < 0.05.
Extended Data Figure 2 Binding of different maltodextrins to the maltose sensor, determination of pore sizes and the relative fraction of open pores, and characterization of the single vesicle fusion assay.
a, Fluorescence emission spectra of the maltose sensor in the absence or presence of the indicated maltodextrin (top). Equilibrium titration of maltodextrin binding to the maltose sensor. The data were fitted with a single site binding equation, using Prism 6 (GraphPad), to determine the dissociation constants. n = 3 independent experiments. Data are presented as mean ± s.d. (bottom). b, Kinetics of maltodextrin binding to the maltose sensor using stopped-flow (top). The observed rate constants (Kobsd) were plotted against maltodextrin concentration. The data were fitted with linear functions, yielding the off- and on-rates for binding of each maltodextrin to the maltose sensor, as follows: 3 ± 1 s−1 and 0.58 ± 0.03 μM−1 s−1 (maltose), 14 ± 1 s−1 and 6.7 ± 0.3 μM−1 s−1 (maltotriose), and 29 ± 9 s−1 and 7.3 ± 0.2 μM−1 s−1 (maltoheptaose) (bottom). n = 3 independent experiments. Data are presented as mean ± s.d. c, The lengths of the three principal axes for each sugar were averaged during 10-ns simulations (left). Error bars indicate s.d. from 1,000 snapshots taken every 10 ps during the simulation. Data are presented as mean ± s.e.m. Representative snapshots of the sugars from the simulations are shown as space-filling models (right). d, Pore sizes were determined from the maltodextrin flux assays shown in Fig. 2c (see Methods). n = 3 independent experiments. e, Representative traces of dithionite quenching experiments using ND3 and ND8. Dithionite was added at the indicated time points during fusion reactions to determine the degree of protection of NBD. The degree of protection was plotted against the incubation time, as shown in Fig. 2d. Similar results were obtained in three independent trials. Quenching by dithionite is much faster than cargo release (for example, Fig. 2b). This is because the kinetics of most of the dithionite quenching that was observed was not a reflection of its influx via fusion pores, as more than 50% of the NBD–PE is on the outer leaflet. It is likely that dithionite can readily enter even small, flickering fusion pores, such as those formed by ND3, because it is smaller (174.11 Da) than the smallest maltodextrin used in this study (maltose; 360.31 Da). Also, the dithionite is present at high concentrations (5 mM). f, Plot of fusion probability observed using the indicated nanodiscs; the black bars indicate experiments in which t-SNARE SUVs were pre-incubated with cd-SYB2 to prevent trans-SNARE pairing. Data are presented as mean ± s.d. g, Histograms of the fluorescence intensities of the tethered t-SNARE vesicles. n = 54 (ND3), 51 (ND5), and 53 (ND7) traces obtained from four independent trials under each condition.
Extended Data Figure 3 Characterization of the nanodisc–BLM system: effect of t-SNARE density and detection of multiple pores.
a, b, Fusion pores were formed using ND5 and BLMs with different t-SNARE densities. t-SNARE density was varied by using SUVs that harboured 100 (SUVt-SNARE (100)), 200 (SUVt-SNARE (200)) or 400 (SUVt-SNARE (400)) copies of the SNAP-25B/syntaxin1A heterodimer per liposome. As SUVt-SNARE (200) and SUVt-SNARE (400) resulted in fusion pores with similar sizes and kinetics properties, SUVt-SNARE (200) was used for all other experiments in this study. n = 5 for SUVt-SNARE (100) and 20 for (SUVt-SNARE (200)); n = 5 for SUVt-SNARE (400). The representative traces (a) correspond to data points demarcated with red arrows in the plot of current against t-SNARE copy number (b). Data are presented as mean ± s.e.m. c, Estimation of the t-SNARE density in the BLMs used to form fusion pores. Typical recording showing that multiple t-SNARE SUVs, bearing a single gramicidin pore, fuse with the planar lipid bilayer. d, Histogram of the number of gramicidin pores formed, as shown in c, from 21 trials. Histogram of the number of gramicidin pores formed (n = 21). e, Multiple pores sometimes form in the nanodisc–BLM assay. e, Example of a recording (SUVt-SNARE (200) and ND5) in which a second pore appeared (top). Current histograms of the recording in the upper panel are shown (bottom). Similar results were obtained in fifteen independent trials.
Extended Data Figure 4 Nanodisc–BLM fusion pore properties at various time points.
a, Typical recording of a fusion pore formed using ND5; this pore eventually closed after ~100 min. Similar results were obtained in eleven independent trials. b, Stability of fusion pores in the nanodisc–BLM assay. Current histograms of nanodisc–BLM assays using SUVt-SNARE (200) and ND0, ND3, ND5, or ND7 at different time points in the recordings. There were no significant differences at the beginning, middle, or end of a recording session, therefore fusion pores are stable. The baseline was also stable over the course of all recordings reported in this study. n = 14 for ND3; n = 20 for ND5 and ND7. For clarity, the closed state is shown in black and the open state is shown in red. In the case of ND0 and ND3, a cyan box is included to mark the appearance of open pores in ND3.
Extended Data Figure 5 Fusion pores formed using 50-nm nanodiscs often dilate.
a–c, Current histograms (left) and representative traces (right) of dilating fusion pores formed using ND3 (a, n = 7), ND5 (b, n = 9) and ND7 (c, n = 10). d, Fraction of time for which pores are open. As fusion pores often dilated, we analysed the currents during an early phase of their initial open state (0.5 s after pore formation). Increasing the copy number of SNAREs per nanodisc resulted in larger pores12 (a–c) that spent more time in the open state (before they dilated; d). Data are presented as mean ± s.e.m. e, A subpopulation of fusion pores formed using 50-nm nanodiscs fails to dilate. Representative traces (left), current (middle) and open dwell time (right) histograms of non-dilating fusion pores (observed in 5 out of 14 trials) formed using 50-nm ND5. These pores exhibit well-defined open and closed states. There is some degree of heterogeneity regarding the v-SNARE copy number per nanodisc9 (Fig. 3c). The non-dilating pores are likely to arise from nanodiscs with the lower v-SNARE densities, consistent with a model in which SNARE density drives dilation19.
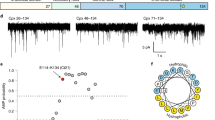
Extended Data Figure 6 Characterization of fusion pores formed by yeast SNAREs.
a, Illustration of pores formed using the yeast SNARE complex comprising Sso1p, the appropriate fragment of Sec9c (residues 401–651), and Snc2p. b, Typical recordings of fusion pores formed using ND0, ND3, ND5, and ND7. c, d, Open dwell-time histogram (c) and a scatter plot of the currents (d) that result from fusion pores formed using ND3 (n = 10), ND5 (n = 14) and ND7 (n = 14). ANOVA P < 0.001; linear trend post hoc P < 0.001. Red arrows in d indicate the representative pores shown in c. Data are presented as mean ± s.e.m. There is a significant increase in pore size and stability as the v-SNARE copy number is increased. The rate constants for pore closure are reported in Extended Data Table 2. ***P < 0.001.
Extended Data Figure 7 Closure of fusion pores caused by cd-SYB2.
a, Fusion pores were first formed using ND5; cd-SYB2 was then added at the indicated concentrations. Partial closure of fusion pores was sometimes observed after addition of cd-SYB2, as shown in the representative current trace. b, Current histogram of all data from the 4 out of 11 trials in which partial closure was observed. In the remaining trials, these sub-conductance states were not observed (Fig. 4a). c, Representative recording (top) and current histogram (bottom) of a pore (ΔΨ = −50 mV) formed using ND7, before and after addition of cd-SYB2 at the indicated concentrations. d, Fraction of closed pores formed using ND5 and ND7 in the presence of 20.25 μM cd-SYB2. Data are presented as mean ± s.e.m.
Extended Data Figure 8 Bovine serum albumin, cd-SYB24A and a C-terminal truncation of SNAP-25B have limited effects on fusion pore current.
Representative traces (top) and current histograms (bottom) of fusion pores before (left) and after (right) addition of bovine serum albumin (BSA) (a) or cd-SYB24A (b). These reagents had no effect on the magnitude of the currents, but cd-SYB24A causes increases in flickering behaviour, probably owing to weak residual t-SNARE binding activity; this effect was limited, as the same concentration of cd-SYB2 completely closed pores under the same conditions. n = 6 for both BSA and cd-SYB24A. c, Fusion pore current is unaffected in SNAP-25B1–197. n = 20 for wild-type SNAP-25B and n = 5 for SNAP-25B1–197. Data are presented as mean ± s.e.m.
Supplementary information
Supplementary Figure 1
This file contains full scans of the blots shown in Figure 1c. (PDF 115 kb)
Source data
Rights and permissions
About this article
Cite this article
Bao, H., Das, D., Courtney, N. et al. Dynamics and number of trans-SNARE complexes determine nascent fusion pore properties. Nature 554, 260–263 (2018). https://doi.org/10.1038/nature25481
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25481
This article is cited by
-
Membrane transformations of fusion and budding
Nature Communications (2024)
-
Mechanisms of SNARE proteins in membrane fusion
Nature Reviews Molecular Cell Biology (2024)
-
Synaptotagmin-7 outperforms synaptotagmin-1 to promote the formation of large, stable fusion pores via robust membrane penetration
Nature Communications (2023)
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
The complexin C-terminal amphipathic helix stabilizes the fusion pore open state by sculpting membranes
Nature Structural & Molecular Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.