Abstract
Brown adipose tissue is a thermogenic organ that dissipates chemical energy as heat to protect animals against hypothermia and to counteract metabolic disease1. However, the transcriptional mechanisms that determine the thermogenic capacity of brown adipose tissue before environmental cold are unknown. Here we show that histone deacetylase 3 (HDAC3) is required to activate brown adipose tissue enhancers to ensure thermogenic aptitude. Mice with brown adipose tissue-specific genetic ablation of HDAC3 become severely hypothermic and succumb to acute cold exposure. Uncoupling protein 1 (UCP1) is nearly absent in brown adipose tissue lacking HDAC3, and there is also marked downregulation of mitochondrial oxidative phosphorylation genes resulting in diminished mitochondrial respiration. Remarkably, although HDAC3 acts canonically as a transcriptional corepressor2, it functions as a coactivator of oestrogen-related receptor α (ERRα) in brown adipose tissue. HDAC3 coactivation of ERRα is mediated by deacetylation of PGC-1α and is required for the transcription of Ucp1, Ppargc1a (encoding PGC-1α), and oxidative phosphorylation genes. Importantly, HDAC3 promotes the basal transcription of these genes independently of adrenergic stimulation. Thus, HDAC3 uniquely primes Ucp1 and the thermogenic transcriptional program to maintain a critical capacity for thermogenesis in brown adipose tissue that can be rapidly engaged upon exposure to dangerously cold temperature.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004)
Guenther, M. G., Barak, O. & Lazar, M. A. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol. Cell. Biol. 21, 6091–6101 (2001)
Chouchani, E. T. et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116 (2016)
Enerbäck, S. et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94 (1997)
Ukropec, J., Anunciado, R. P., Ravussin, Y., Hulver, M. W. & Kozak, L. P. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J. Biol. Chem. 281, 31894–31908 (2006)
Bal, N. C. et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579 (2012)
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015)
Long, J. Z. et al. The secreted enzyme PM20D1 regulates lipidated amino acid uncouplers of mitochondria. Cell 166, 424–435 (2016)
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013)
Loft, A., Forss, I. & Mandrup, S. Genome-wide insights into the development and function of thermogenic adipocytes. Trends Endocrinol. Metab. 28, 104–120 (2017)
Chen L.-f., Fischle, W., Verdin, E. & Greene, W. C. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293, 1653–1657 (2001)
Bhaskara, S. et al. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol. Cell 30, 61–72 (2008)
Sun, Z. et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat. Med. 18, 934–942 (2012)
Mullican, S. E. et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 25, 2480–2488 (2011)
Hoeksema, M. A. et al. Targeting macrophage histone deacetylase 3 stabilizes atherosclerotic lesions. EMBO Mol. Med. 6, 1124–1132 (2014)
Razidlo, D. F. et al. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS ONE 5, e11492 (2010)
Alenghat, T. et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature 504, 153–157 (2013)
Montgomery, R. L. et al. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J. Clin. Invest. 118, 3588–3597 (2008)
Sun, Z. et al. Diet-induced lethality due to deletion of the Hdac3 gene in heart and skeletal muscle. J. Biol. Chem. 286, 33301–33309 (2011)
Rada-Iglesias, A. et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283 (2011)
Core, L. J., Waterfall, J. J. & Lis, J. T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008)
Fang, B. et al. Circadian enhancers coordinate multiple phases of rhythmic gene transcription in vivo. Cell 159, 1140–1152 (2014)
Wang, D. et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474, 390–394 (2011)
Gerhart-Hines, Z. et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 503, 410–413 (2013)
Giguère, V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr. Rev. 29, 677–696 (2008)
Soccio, R. E. et al. Genetic variation determines PPARγ function and anti-diabetic drug response in vivo. Cell 162, 33–44 (2015)
Rajakumari, S. et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 17, 562–574 (2013)
Dixen, K. et al. ERRγ enhances UCP1 expression and fatty acid oxidation in brown adipocytes. Obesity (Silver Spring) 21, 516–524 (2013)
Rodgers, J. T., Lerin, C., Gerhart-Hines, Z. & Puigserver, P. Metabolic adaptations through the PGC-1α and SIRT1 pathways. FEBS Lett. 582, 46–53 (2008)
Uldry, M. et al. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 3, 333–341 (2006)
Mullican, S. E. et al. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol. Endocrinol. 27, 127–134 (2013)
Luo, J. et al. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol. Cell. Biol. 23, 7947–7956 (2003)
Cannon, B. & Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 214, 242–253 (2011)
Pecinová, A., Drahota, Z., Nůsková, H., Pecina, P. & Houštěk, J. Evaluation of basic mitochondrial functions using rat tissue homogenates. Mitochondrion 11, 722–728 (2011)
Picard, M. et al. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS ONE 6, e18317 (2011)
Houste˘k, J., Cannon, B. & Lindberg, O. Gylcerol-3-phosphate shuttle and its function in intermediary metabolism of hamster brown-adipose tissue. Eur. J. Biochem. 54, 11–18 (1975)
Wang, H. et al. Resveratrol rescues kidney mitochondrial function following hemorrhagic shock. Shock 44, 173–180 (2015)
Porter, C. et al. Human and mouse brown adipose tissue mitochondria have comparable UCP1 function. Cell Metab. 24, 246–255 (2016)
Feng, D. et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 331, 1315–1319 (2011)
Harms, M. J. et al. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 19, 593–604 (2014)
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013)
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014)
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010)
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013)
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44 (W1), W90–W97 (2016)
Eyre, T. A. et al. The HUGO Gene Nomenclature Database, 2006 updates. Nucleic Acids Res. 34, D319–D321 (2006)
Kent, W. J., Zweig, A. S., Barber, G., Hinrichs, A. S. & Karolchik, D. BigWig and BigBed: enabling browsing of large distributed datasets. Bioinformatics 26, 2204–2207 (2010)
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010)
Sequence Read Archive Submissions Staff. Using the SRA Toolkit to convert .sra files into other formats. SRA Knowledge Base https://www.ncbi.nlm.nih.gov/books/NBK158900/ (2011)
Thorvaldsdóttir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 (2013)
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011)
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009)
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010)
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012)
Harms, M. J. et al. PRDM16 binds MED1 and controls chromatin architecture to determine a brown fat transcriptional program. Genes Dev. 29, 298–307 (2015)
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17, 10 (2011)
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015)
Robinson, M. D. & Smyth, G. K. Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 9, 321–332 (2008)
Step, S. E. et al. Anti-diabetic rosiglitazone remodels the adipocyte transcriptome by redistributing transcription to PPARγ-driven enhancers. Genes Dev. 28, 1018–1028 (2014)
Acknowledgements
We thank the Next-Generation Sequencing Core and the Mouse Phenotyping, Physiology and Metabolism Core of the Penn Diabetes Research Center (National Institutes of Health (NIH) P30 DK19525). This work was supported by NIH R01 DK45586 (M.A.L.), NIH F30 DK104513 (M.J.E.), NIH R01 DK106027 (K.-J.W.), and the JPB Foundation.
Author information
Authors and Affiliations
Contributions
M.J.E. and M.A.L. conceived the project, designed experiments, analysed results, and wrote the manuscript; M.J.E. performed animal experiments, immunoblots, RNA-seq, and ChIP–seq; M.J.E. and J.J. performed GRO-seq; H.-W.L. and K.-J.W. performed bioinformatic analyses; C.A.S. and M.J.E. performed mitochondrial assays. M.J.E. and H.J.R. performed cellular experiments. M.A. performed endogenous co-immunoprecipitation. D.J.S. performed H3/H3Kme1 ChIP–seq. L.C.P. and E.R.B. provided animal husbandry and technical assistance. T.M., Z.G.-H., P.S., J.A.B. and L.P. provided reagents and experimental design. All authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks A. Vidal-Puig and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Ablation of HDAC3 in adipose tissue depots.
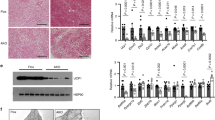
a, Immunoblot analysis of interscapular BAT, iWAT, and eWAT of Adipoq-cre HDAC3 KO and control littermates, or Ucp1-cre HDAC3 KO and control littermates maintained at 22 °C (n = 2, all groups) demonstrating tissue-specific conditional KO of HDAC3. b–d, Interscapular BAT mass (b), relative BAT mitochondrial number (c), and total body mass (d) from Adipoq-cre HDAC3 KO and Ucp1-cre HDAC3 KO versus control littermates maintained at 22 °C (n = 13 Adipoq-cre, n = 9 control; n = 9 Ucp1-cre, n = 10 control). e, Representative haematoxylin and eosin (H&E) staining of inguinal white adipose from 10- to 12-week-old Adipoq-cre HDAC3 KO, Ucp1-cre HDAC3 KO, Ucp1 KO, or control mice housed at 22 °C. Lower panels show higher magnification of the boxed areas in upper panels. Scale bars, 100 μm. Data are represented as mean ± s.e.m.
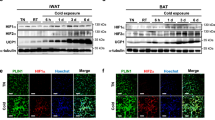
Extended Data Figure 2 BAT HDAC3 is required for cold-mediated induction of Ucp1 expression, and HDAC3 expression is not altered by acute cold.
a, b, BAT Ucp1 mRNA levels following a 3 h exposure to 4 °C (from 22 °C) versus control littermates maintained at 22 °C in (a) Adipoq-cre HDAC3 KO versus control (n = 5, 5, per temperature) and (b) Ucp1-cre HDAC3 KO versus control (n = 5, 5, per temperature). c, d, iWAT Ucp1 mRNA levels following 3 h exposure to 4 °C, versus control littermates maintained at 22 °C in (c) Adipoq-cre HDAC3 KO versus control (n = 5, 5, per temperature) and (d) Ucp1-cre HDAC3 KO versus control (n = 5, 5, per temperature). e, BAT HDAC3 mRNA expression levels following a 3 h exposure to 4 °C (from 22 °C) versus control littermates maintained at 22 °C (n = 5, 5, per temperature). f, BAT HDAC3 protein levels following 3 h acute cold exposure at 4 °C (from 22 °C) versus control littermates maintained at 22 °C. VCL, vinculin. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 as determined by a two-way ANOVA with Holm–Šidák’s post hoc test (a–d) or two-tailed Student’s t-test (e). Data are represented as mean ± s.e.m.
Extended Data Figure 3 HDAC3 is neither induced nor required for brown adipogenesis, but required for cell-autonomous Ucp1 expression.
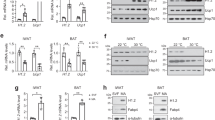
a, Gene expression spanning differentiation of cultured WT primary brown adipocytes (n = 5 replicates per time point). b, Depletion of HDAC3 in day 8 cultured mature brown adipocytes after addition of 2 μm 4-hydroxytamoxifen (4-OHT) during days 0–2 of differentiation to Rosa26-CreER-positive (HDAC3 KO) and Rosa26-CreER-negative (control) cells derived from littermates (n = 3, 3). c, Adipocyte-specific gene expression in cultured primary brown adipocytes after depletion of HDAC3 versus control (n = 3, 3). d, Assessment of lipid accumulation (evaluated by Oil Red-O staining) in cultured HDAC3 KO versus control primary brown adipocytes. e, Ucp1 mRNA expression in cultured primary brown adipocytes after depletion of HDAC3 versus control (n = 3, 3). f, UCP1 protein expression in cultured primary brown adipocytes after depletion of HDAC3 versus control. (n = 3, 3). VCL, vinculin. g, Ucp1 mRNA expression in cultured primary brown adipocytes after depletion of HDAC3 versus control and treated with vehicle (ethanol) or isoproterenol (1 μm) for 3 h (n = 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, as determined by a two-tailed Student’s t-test (b, c, e) or by a two-way ANOVA with Holm–Šidák’s post hoc test (g). Data are represented as mean ± s.e.m.
Extended Data Figure 4 HDAC3 is required for expression of mitochondrial OXPHOS and TCA cycle genes.
a, Bioinformatic extension of identified Gene Ontology categories (Fig. 2c) to all oxidative phosphorylation and TCA cycle genes as retrieved by the HUGO gene nomenclature database. *Gene expression change in RNA-seq dataset with an FDR < 0.01. b, c, RT–qPCR verification of gene expression changes highlighted in Fig. 2d: b, Adipoq-cre HDAC3 KO versus control littermates at 29 °C (upper, n = 9, 6) and 22 °C (lower, n = 9, 7); c, Ucp1-cre HDAC3 KO versus control littermates at 29 °C (upper, n = 5, 6) and 22 °C (lower, n = 5, 7). *P < 0.05, **P < 0.01, ***P < 0.001, as determined by a two-tailed Student’s t-test. Data are represented as mean ± s.e.m.
Extended Data Figure 5 Metabolic studies of Adipoq-cre and Ucp1-cre HDAC3 KO mouse models.
a, b, NMR analysis of body composition: a, Adipoq-cre HDAC3 KO mice versus control littermates (n = 8, 11); b, Ucp1-cre HDAC3 KO mice versus control littermates (n = 7, 9). c–n, CLAMS metabolic cage analysis. c, d, Oxygen consumption (VO2): c, Adipoq-cre HDAC3 KO versus control littermates (n = 6, 5); d, Ucp1-cre HDAC3 KO versus control littermates (n = 6, 6). e, f, ANCOVA of VO2 (linear regression analysis of total body mass and oxygen consumption): e, Adipoq-cre HDAC3 KO versus control littermates (n = 6, 5); f, Ucp1-cre HDAC3 KO versus control littermates (n = 6, 6). g, h, Respiratory exchange ratio (RER): g, Adipoq-cre HDAC3 KO versus control littermates (n = 6, 5); h, Ucp1-cre HDAC3 KO versus control littermates (n = 6, 6). i, j, Heat measurements (kcal h−1): i, Adipoq-cre HDAC3 KO versus control littermates (n = 6, 5); j, Ucp1-cre HDAC3 KO versus control littermates (n = 6, 6). k, l, Food intake: k, Adipoq-cre HDAC3 KO versus control littermates (n = 6, 5); l, Ucp1-cre HDAC3 KO versus control littermates (n = 6, 6). m, n, Physical activity: m, Adipoq-cre HDAC3 KO versus control littermates (n = 6, 5); n, Ucp1-cre HDAC3 KO versus control littermates (n = 6, 6). P values shown in italics. CLAMS data are graphed as rolling averages. NS, not significant, *P < 0.05 as determined by a two-tailed Student’s t-test (a–d, g–n) or ANCOVA (e, f). Data are represented as mean ± s.e.m.
Extended Data Figure 6 Effect of high-fat diet on Adipoq-cre and Ucp1-cre HDAC3 KO mouse models.
Twelve-week-old weight-matched HDAC3 KO and control littermates were fed high-fat diet (HFD) for 12 weeks. a, Weekly body weights, (n = 8 Adipoq-cre, n = 10 control). b, Body composition analysis by NMR (n = 8 Adipoq-cre, n = 10 control). c, Weekly body weights, (n = 7 Ucp1-cre, n = 7 control). d, Body composition analysis by NMR (n = 7 Ucp1-cre, n = 7 control). e, RT–qPCR of BAT HDAC3 mRNA expression after 12 weeks high-fat diet versus controls fed with regular chow (n = 7, 5, respectively). Data are represented as mean ± s.e.m.
Extended Data Figure 7 Transcriptional role of HDAC3 and ERRα in BAT.
a, Heat map demonstrating correlation of RNA-seq and GRO-seq data. Differentially expressed genes in RNA-seq or GRO-seq data were sorted by log2(fold change) in RNA-seq. FC, fold change. b, De novo motif enrichment at repressed eRNAs in Adipoq-cre, HDAC3 KO mice versus control littermates (n = 10, 10; pooled biological replicates per library) maintained at 22 °C and ranked by P value. c, Endogenous HDAC3 co-immunoprecipitation of ERRα in differentiated mature brown adipocytes. d, e, RT–qPCRs of BAT Ucp1 eRNA expression and (f) Ucp1 mRNA at 22 °C and 29 °C in Adipoq-cre and Ucp1-cre HDAC3 KO mice versus control littermates, 29 °C (n = 9 Adipoq-cre, n = 6 control; n = 5 Ucp1-cre, n = 6 control) and 22 °C (n = 9 Adipoq-cre, n = 7 control; n = 5 Ucp1-cre, n = 7 control). g, ChIP–qPCR of ERRα at Ucp1 enhancers in Adipoq-cre HDAC3 KO versus control littermates (n = 3, 3) adapted to 29 °C. h, RT–qPCR of ERRα and Ucp1 mRNA expression and (i) Ucp1 eRNA expression in ERRα KO BAT versus control littermates (n = 8, 7). j, RT–qPCR analysis of ERRα and Ucp1 mRNA expression in mature brown adipocytes after siRNA-mediated knockdown of ERRα versus scramble 72 h after transfection (n = 3, 3). *P < 0.05, **P < 0.01, ***P < 0.001 as determined by a two-tailed Student’s t-test (d–e, g–j), a two-way ANOVA with Holm–Šidák’s post hoc test (f). P values for motif enrichment as determined by binomial test (b). Data are represented as mean ± s.e.m.
Extended Data Figure 8 Role of HDAC3 on PGC-1α acetylation and function.
a, Co-immunoprecipitation of HDAC3 and PGC-1α with ERRα from HEK-293FT cells. b, Luciferase reporter assay of transcription driven by the major Ucp1 enhancer (−6 kb) after transfection of ERRα, PGC-1α, GCN5, and/or HDAC3 (n = 3 replicates per condition). c, d, Primary brown pre-adipocytes from Rosa26-CreER-positive Hdac3f/f and Hdac3f/f control littermates transduced with MSCV retroviruses: control, PGC-1α WT, or non-acetylatable PGC-1α R13 mutant, treated with 2 μm 4-hydroxytamoxifen during days 0–2 of differentiation to deplete HDAC3, and studied at day 8 of differentiation. c, Immunoblot analysis of exogenous PGC-1α expression in primary brown adipocytes (n = 2 replicates pooled per lane). d, RT–qPCR analysis of Ucp1 and Fasn expression in control and HDAC3 KO primary brown adipocytes following transduction with MSCV-Control (n = 4 control, 3 HDAC3 KO), MSCV-PGC-1α WT (n = 4 control, 4 HDAC3 KO), or MSCV-PGC-1α R13 (non-acetylatable mutant) (n = 3 Control, 4 HDAC3 KO). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, as determined by a one-way ANOVA with a Tukey’s post hoc test (b, d). Data are represented as mean ± s.e.m.
Extended Data Figure 9 HDAC3 and ERRα activate Ppargc1b enhancers and transcription.
a, Genome browser tracks of the Ppargc1b locus highlighting GRO-seq and ChIP–seq data from HDAC3 KO and control BAT (y axis scales, normalized reads, reads per million) demonstrating co-binding of HDAC3, ERRα, and NCoR at functional enhancers. b, BAT Ppargc1b mRNA levels in Adipoq-cre HDAC3 KO BAT versus control littermates (29 °C: n = 9, 6; 22 °C: n = 9, 7). c, d, RT–qPCR of eRNAs found at HDAC3 and ERRα enhancers in Adipoq-cre HDAC3 KO BAT versus control littermates (29 °C: n = 9, 6; 22 °C: n = 9, 7) and Ucp1-cre HDAC3 KO BAT versus control littermates (29 °C: n = 5, 6; 22 °C: n = 5, 7). e, RT–qPCR analysis of Ucp1 mRNA expression in mature brown adipocytes after combinatorial siRNA knockdown of Pgc-1α, Pgc-1β, and/or ERRα versus scramble siRNA (n = 5 replicates per condition). Statistical analysis performed among groups transfected with siRNAs. f, Quantification of Ucp1 and Pgc-1α nascent gene body transcription (GRO-seq) at 22 °C and 29 °C in Adipoq-cre HDAC3 KO BAT versus control littermates (n = 10, 10, pooled biological replicates per library). *P < 0.05, **P < 0.01, ***P < 0.001, as determined by a two-tailed Student’s t-test (b–d), one-way ANOVA with a Holm–Šidák post hoc test (e), or an exact test (performed in EdgeR). Data are represented as mean ± s.e.m.
Supplementary information
Supplementary Data
This file contains a PDF of figures and extended data figures for immunoblots and uncropped gels. (PDF 3147 kb)
Supplementary Table
This file contains a PDF of a table of primers that were used for real-time qPCR, ChIP-qPCR, and PCR cloning. (PDF 79 kb)
Source data
Rights and permissions
About this article
Cite this article
Emmett, M., Lim, HW., Jager, J. et al. Histone deacetylase 3 prepares brown adipose tissue for acute thermogenic challenge. Nature 546, 544–548 (2017). https://doi.org/10.1038/nature22819
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22819
This article is cited by
-
Histone proteoform analysis reveals epigenetic changes in adult mouse brown adipose tissue in response to cold stress
Epigenetics & Chromatin (2024)
-
Nuclear receptor corepressors non-canonically drive glucocorticoid receptor-dependent activation of hepatic gluconeogenesis
Nature Metabolism (2024)
-
Targeting adipocyte ESRRA promotes osteogenesis and vascular formation in adipocyte-rich bone marrow
Nature Communications (2024)
-
SMYD3: a new regulator of adipocyte precursor proliferation at the early steps of differentiation
International Journal of Obesity (2024)
-
Ubiquitin ligase RNF20 coordinates sequential adipose thermogenesis with brown and beige fat-specific substrates
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.