Abstract
In humans and other mammalian species, lesions in the preoptic area of the hypothalamus cause profound sleep impairment1,2,3,4,5, indicating a crucial role of the preoptic area in sleep generation. However, the underlying circuit mechanism remains poorly understood. Electrophysiological recordings and c-Fos immunohistochemistry have shown the existence of sleep-active neurons in the preoptic area, especially in the ventrolateral preoptic area and median preoptic nucleus6,7,8,9. Pharmacogenetic activation of c-Fos-labelled sleep-active neurons has been shown to induce sleep10. However, the sleep-active neurons are spatially intermingled with wake-active neurons6,7, making it difficult to target the sleep neurons specifically for circuit analysis. Here we identify a population of preoptic area sleep neurons on the basis of their projection target and discover their molecular markers. Using a lentivirus expressing channelrhodopsin-2 or a light-activated chloride channel for retrograde labelling, bidirectional optogenetic manipulation, and optrode recording, we show that the preoptic area GABAergic neurons projecting to the tuberomammillary nucleus are both sleep active and sleep promoting. Furthermore, translating ribosome affinity purification and single-cell RNA sequencing identify candidate markers for these neurons, and optogenetic and pharmacogenetic manipulations demonstrate that several peptide markers (cholecystokinin, corticotropin-releasing hormone, and tachykinin 1) label sleep-promoting neurons. Together, these findings provide easy genetic access to sleep-promoting preoptic area neurons and a valuable entry point for dissecting the sleep control circuit.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Von Economo, C. Sleep as a problem of localization. J. Nerv. Ment. Dis. 71, 249–259 (1930)
Nauta, W. J. Hypothalamic regulation of sleep in rats; an experimental study. J. Neurophysiol. 9, 285–316 (1946)
McGinty, D. J. & Sterman, M. B. Sleep suppression after basal forebrain lesions in the cat. Science 160, 1253–1255 (1968)
Sallanon, M. et al. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience 32, 669–683 (1989)
Lu, J., Greco, M. A., Shiromani, P. & Saper, C. B. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J. Neurosci. 20, 3830–3842 (2000)
Szymusiak, R., Alam, N., Steininger, T. L. & McGinty, D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 803, 178–188 (1998)
Takahashi, K., Lin, J. S. & Sakai, K. Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience 161, 269–292 (2009)
Gong, H. et al. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J. Physiol. (Lond.) 556, 935–946 (2004)
Sherin, J. E., Shiromani, P. J., McCarley, R. W. & Saper, C. B. Activation of ventrolateral preoptic neurons during sleep. Science 271, 216–219 (1996)
Zhang, Z. et al. Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists. Nat. Neurosci. 18, 553–561 (2015)
Sherin, J. E., Elmquist, J. K., Torrealba, F. & Saper, C. B. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 18, 4705–4721 (1998)
Steininger, T. L., Gong, H., McGinty, D. & Szymusiak, R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J. Comp. Neurol. 429, 638–653 (2001)
Cetin, A. & Callaway, E. M. Optical control of retrogradely infected neurons using drug-regulated “TLoop” lentiviral vectors. J. Neurophysiol. 111, 2150–2159 (2014)
Berndt, A. et al. Structural foundations of optogenetics: determinants of channelrhodopsin ion selectivity. Proc. Natl Acad. Sci. USA 113, 822–829 (2016)
Beier, K. T. et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634 (2015)
Heiman, M. et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell 135, 738–748 (2008)
Mansbach, R. S. & Lorenz, D. N. Cholecystokinin (CCK-8) elicits prandial sleep in rats. Physiol. Behav. 30, 179–183 (1983)
Kimura, M. et al. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol. Psychiatry 15, 154–165 (2010)
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011)
Sternson, S. M. & Roth, B. L. Chemogenetic tools to interrogate brain functions. Annu. Rev. Neurosci. 37, 387–407 (2014)
Gaus, S. E., Strecker, R. E., Tate, B. A., Parker, R. A. & Saper, C. B. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience 115, 285–294 (2002)
Zhang, G., Wang, L., Liu, H. & Zhang, J. Substance P promotes sleep in the ventrolateral preoptic area of rats. Brain Res. 1028, 225–232 (2004)
Greco, M. A. et al. Opioidergic projections to sleep-active neurons in the ventrolateral preoptic nucleus. Brain Res. 1245, 96–107 (2008)
Okaty, B. W., Sugino, K. & Nelson, S. B. A quantitative comparison of cell-type-specific microarray gene expression profiling methods in the mouse brain. PLoS ONE 6, e16493 (2011)
Weber, F. et al. Control of REM sleep by ventral medulla GABAergic neurons. Nature 526, 435–438 (2015)
Xu, M. et al. Basal forebrain circuit for sleep-wake control. Nat. Neurosci. 18, 1641–1647 (2015)
Jego, S. et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 16, 1637–1643 (2013)
Van Dort, C. J. et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc. Natl Acad. Sci. USA 112, 584–589 (2015)
Hayashi, Y. et al. Cells of a common developmental origin regulate REM/non-REM sleep and wakefulness in mice. Science 350, 957–961 (2015)
Anaclet, C. et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat. Neurosci. 17, 1217–1224 (2014)
Franklin, K. B. J. & Paxinos, G. in The Mouse Brain in Stereotaxic Coordinates 3rd edn, 31–52 (Academic, 2007)
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011)
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010)
Krashes, M. J. et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242 (2014)
Terao, A., Greco, M. A., Davis, R. W., Heller, H. C. & Kilduff, T. S. Region-specific changes in immediate early gene expression in response to sleep deprivation and recovery sleep in the mouse brain. Neuroscience 120, 1115–1124 (2003)
Borbély, A. A., Tobler, I. & Hanagasioglu, M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav. Brain Res. 14, 171–182 (1984)
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2011)
Schmitzer-Torbert, N., Jackson, J., Henze, D., Harris, K. & Redish, A. D. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131, 1–11 (2005)
Knight, Z. A. et al. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 151, 1126–1137 (2012)
Mi, H., Muruganujan, A., Casagrande, J. T. & Thomas, P. D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protocols 8, 1551–1566 (2013)
Sugino, K. et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat. Neurosci. 9, 99–107 (2006)
Hempel, C. M., Sugino, K. & Nelson, S. B. A manual method for the purification of fluorescently labeled neurons from the mammalian brain. Nat. Protocols 2, 2924–2929 (2007)
Tasic, B. et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 19, 335–346 (2016)
Zhang, S. et al. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665 (2014)
Lambolez, B., Audinat, E., Bochet, P., Crépel, F. & Rossier, J. AMPA receptor subunits expressed by single Purkinje cells. Neuron 9, 247–258 (1992)
Acknowledgements
We thank J. Cox, L. Pinto for help with sleep recordings; A. Popescu for help with optrode recordings; C. Ma for help with FISH; D. Leib, C. Zimmerman, and D. Estandian for discussions on TRAP; K. Kao, G. Daly, M. Bikov, F. Virani, and G. Carrillo for technical assistance; and C. Koch for supporting the collaboration with the Allen Institute for Brain Science. This work was supported by a Davis Postdoctoral Fellowship (S.C.), Tourette Syndrome Association Grant (S.C.), EMBO Long-term Fellowship (F.W.), Human Frontier Science Program Fellowship (F.W.), and Howard Hughes Medical Institute (Y.D., L.L.).
Author information
Authors and Affiliations
Contributions
S.C. and Y.D. conceived and designed the study, and wrote the paper. S.C. performed most of the experiments. F.W. wrote the programs for data analysis and sleep recording, and S.C. and F.W. analysed the data. P.Z. performed slice recordings. C.L.T. and Z.A.K. constructed the herpes simplex virus (HSV virus), and performed TRAP experiments. T.N., B.T., and H.Z. performed single-cell RNA-seq. K.T.B. and L.L. provided cTRIO and axon arborization analysis reagents and performed part of the input tracing experiments. N.H. and Z.Z. performed FISH. W.C.C. and J.P.D. performed part of the sleep recording. M.J.K. generated PDYN-IRES-Cre mice. S.Y. and A.C. constructed the lentivirus. Y.D. supervised all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks M. Halassa, T. Kilduff, A. Yamanaka and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
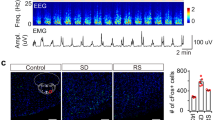
Extended Data Figure 1 Overlap of c-Fos staining of sleep-active POA neurons with retrograde labelling from several brain regions and with GAD1–GFP or VGLUT2–GFP.
a, Overlap between c-Fos expression induced by sleep rebound (Sleep, n = 5 mice) or sleep deprivation (Wake, n = 3) and retrobead (RB) labelling from the TMN, dorsomedial hypothalamus (DMH, n = 3), or ventrolateral periaqueductal grey (vlPAG, n = 3). Mouse brain figure adapted with permission from ref. 31. Left column, representative images showing retrobead injection sites (scale bars, 500 μm). Percentage of c-Fos+ neurons containing retrobead and percentage of retrobead-containing cells that were c-Fos+ were both significantly different among target regions (P < 0.0001 and P = 0.0004, one-way analysis of variance followed by Dunnett’s post-hoc test). Many retrobead-labelled neurons from TMN expressed c-Fos following sleep but not following wake. b, Overlap between c-Fos expression induced by sleep rebound and GAD1–GFP (top, five mice) or VGLUT2–GFP (bottom, three mice). Many c-Fos+ cells were GAD1+ (arrowheads). Error bar, ±s.e.m.
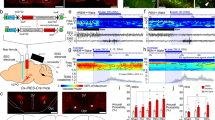
Extended Data Figure 2 Innervation of histamine neurons in the TMN by GABAergic neurons in the POA and overlap of lentivirus labelling of GABAPOA→TMN neurons with GAD expression and with c-Fos labelling after sleep rebound.
a, In GAD2-Cre mice injected with AAV-DIO-ChR2–eYFP in the POA (left), ChR2–eYFP-expressing axons (green) are observed in the TMN area (red, histidine decarboxylase (HDC)). Mouse brain figure adapted with permission from ref. 31. b, Inhibitory postsynaptic current (red) recorded in an example TMN histamine neuron, evoked by optogenetic activation of POA GABAergic axons. Light-evoked responses (red) were blocked by bicuculline (black). Blue tick, laser stimulus. c, Amplitudes and latencies of inhibitory postsynaptic currents recorded from TMN histamine neurons (n = 7, from two mice). Each symbol represents data from one cell. Error bar, ±s.e.m. d, Single-cell RT–PCR identification of HDC-expressing histamine neurons. e, Schematic of RV-mediated trans-synaptic retrograde tracing from TMN histamine neurons. Fluorescence image of the TMN shows starter cells (yellow, expressing both GFP and mCherry). Inset, enlarged view of the region in white box. f, Left, fluorescence image showing input neurons in the POA. Right, enlarged view of the region in white box, showing RV–GFP labelling (green) and GAD1/2 expression (red, FISH for mRNA encoding GAD1/2). Arrowheads, RV labelled cells that are GAD1/2+. 79.0 ± 1.4% of RV–GFP labelled neurons contained mRNA encoding GAD1/2 (n = 2 mice). g, Overlap between c-Fos expression induced by sleep rebound and RV–GFP labelling. Left, fluorescence image showing input neurons in the POA. Right, enlarged view of the region in white box, showing RV–GFP labelling and c-Fos expression; 46.9 ± 1.9% of RV–GFP labelled neurons expressed c-Fos (n = 6 mice). h, Expression of eYFP or ChR2–eYFP in the POA induced by injecting rEIAV-DIO-TLoop-nls–eYFP or rEIAV-DIO-TLoop-ChR2–eYFP into the TMN of GAD2-Cre mice and their overlap with GAD1/2 expression (FISH for mRNA encoding GAD1/2). Arrowheads indicate cells co-labelled with GAD-FISH probe and eYFP or ChR2–eYFP; 96.2 ± 1.4% of eYFP-labelled neurons and 95.4 ± 3.7% of ChR2–eYFP-labelled neurons contained mRNA encoding GAD1/2 (n = 4, 5). i, Expression of ChR2–eYFP in the POA induced by injecting rEIAV-DIO-TLoop-ChR2–eYFP into the TMN of a GAD2-Cre mouse and its overlap with c-Fos expression following sleep rebound (arrowheads).
Extended Data Figure 3 Effect of optogenetic activation of GABAPOA→TMN neurons at low frequencies and effect of laser stimulation in GABAPOA→TMN–eYFP control mice.
a, Similar to Fig. 1, rEIAV-DIO-TLoop-ChR2–eYFP was injected into the TMN of GAD2-Cre mice and an optic fibre was implanted into the POA for optogenetic stimulation. Mouse brain figure adapted with permission from ref. 31. b, Percentage of time the mice spent in wake, NREM, or REM state before, during, and after laser stimulation (blue shading, 5 Hz, 120 s), averaged from five mice (P < 0.0001 for wake, REM, and NREM, bootstrap). c, Similar to b, but with 2 Hz stimulation (P < 0.0001 for wake and REM, P = 0.002 for NREM, bootstrap, n = 5 mice). d, Similar to a, after rEIAV-DIO-TLoop-nls–eYFP injection. e, Effect of 10 Hz stimulation in eYFP control mice. Shown is the percentage of time in wake, NREM, or REM state before, during, and after laser stimulation (blue shading, 10 Hz, 120 s), averaged from eight mice (P = 0.18, 0.84, and 0.35 for REM, NREM, and wake respectively, bootstrap). f, Effect of constant light stimulation (blue shading, constant light, 60 s), averaged from five mice (P = 0.57, 0.27, and 0.73 for REM, NREM, and wake, bootstrap). Shading for each trace, 95% confidence interval.
Extended Data Figure 4 Optogenetic manipulation of axon projections of GABAPOA neurons to TMN, dorsomedial hypothalamus, and habenula, and effect of anti-histamine on optogenetic activation of the TMN axon projections.
a, AAV-DIO-ChR2–eYFP or AAV-DIO-iC++–eYFP was injected into the POA of GAD2-Cre mice and an optic fibre was implanted into the TMN for optogenetic activation/inhibition. Mouse brain figure adapted with permission from ref. 31. b, Percentage of time in wake, NREM, or REM state before, during, and after laser stimulation (blue shading, 10 Hz, 120 s) in mice expressing ChR2, averaged from nine mice (P < 0.0001 for wake, REM and NREM, bootstrap). Shading for each trace, 95% confidence interval. c, Similar to b, but in mice expressing iC++ (blue shading: constant light, 60 s), averaged from four mice (P < 0.0001 for wake and NREM, P = 0.004 for REM, bootstrap). d, Schematic for optogenetic activation of POA GABAergic projection to the habenula (Hb). e, Similar to b, for activating POA→habenula projection (P = 0.28, 0.35 and 0.72 for REM, wake and NREM, bootstrap, n = 3 mice). f, Schematic for optogenetic activation of POA GABAergic projection to the dorsomedial hypothalamus. g, Similar to b, for POA→dorsomedial hypothalamus activation (P = 0.02, 0.12, and 0.09 for wake, REM, and NREM, n = 3 mice). h, Left, schematic for optogenetic activation of POA GABAergic projection to the TMN without drug treatment. Right, percentage of time in wake, NREM, or REM state before, during, and after laser stimulation (blue shading, 10 Hz, 120 s) in mice with no drug (effect of laser: P < 0.001 for wake, REM, and NREM, bootstrap; n = 14 mice). i, Similar to h, but after intraperitoneal injection of triprolidine (20 mg per kg (body weight); effect of laser: P = 0.21, 0.84, 0.57 for wake, REM, and NREM, n = 5 mice). j, Percentage of time in NREM, REM, or wake state before and during laser stimulation in no drug and triprolidine groups (120 s periods before and during laser stimulation, *P < 0.05, **P < 0.01, ***P < 0.001; NS, P > 0.05, signed rank test between before and during laser, rank sum test between no drug and triprolidine for the period before laser stimulation). k, Laser-induced change in the percentage of each state (difference between the 120 s periods before and during laser stimulation, *P < 0.05, rank sum test). Error bar, ±s.e.m.
Extended Data Figure 5 Effect of laser stimulation on transition probability between each pair of brain states in GABAPOA→TMN-ChR2, GABAPOA→TMN-Ctrl, and GABAPOA-ChR2 mice.
a, Schematic showing transition probability calculation. To calculate the transition probability at a given time bin (i), we first identified all the trials (n) in which the animal was in state X (X could be wake, NREM, or REM) in the preceding time bin (i − 1). Among these n trials, we identified the subset of trials (m) in which the animal transitioned into state Y in the current time bin (i). The X→Y transition probability for time bin i was computed as m/n. b, Transition probability within each 10 s period in GABAPOA→TMN-ChR2 mice (n = 9). Shown in each bar is the transition probability averaged across six consecutive 10 s bins within each 60 s. Error bar, 95% confidence interval (bootstrap). The baseline transition probability (grey dashed line) was averaged across all time bins after excluding the laser stimulation period. Direct wake→REM and REM→NREM transitions were not observed and the corresponding plots were omitted. Magenta asterisk (*)/green hash symbol (#) indicates significant increase/decrease in transition probability during laser stimulation compared with the baseline (P < 0.05, bootstrap). Top right diagram indicates transition probabilities that are significantly increased (magenta), decreased (green), or unaffected (black) by laser stimulation. c, Transition probability in control mice (n = 8). The probability during laser stimulation was not significantly different from baseline for any transition. d, Transition probability in GABAPOA-ChR2 mice (n = 5).
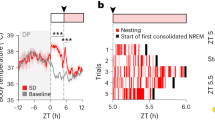
Extended Data Figure 6 Optogenetic identification of GABAPOA→TMN neurons, firing rates of unidentified POA neurons, and firing rate dynamics of identified GABAPOA→TMN neurons during NREM sleep.
a, Distribution of delays in laser-evoked spiking for all identified neurons. Delay was defined as timing of the first spike after each laser pulse. b, Distribution of correlation coefficient between laser-evoked and spontaneous spike waveforms for all identified neurons. c, Firing rates of unidentified units in the three brain states. Each line represents data from one neuron. Grey bar represents average over units (n = 51, from 11 mice). Error bar, ±s.e.m. d, Firing rate change of identified GABAPOA→TMN neurons during each NREM episode. Top: mean EEG power spectrogram from start to end of each NREM episode. Bottom: mean firing rate of the recorded neurons. Left: average across all NREM episodes. Each NREM period was divided into ten time bins (temporally normalized). The firing rate of each neuron was z-scored and averaged across all recorded NREM episodes. Solid line, mean of 17 neurons; shading, ±s.e.m. To test significance of the firing rate increase during NREM episodes, for each unit we measured the slope of its mean firing rate versus time after NREM onset, as quantified by a linear fit. Across the 17 units recorded, the increase (slope > 0) was significant (P = 1.0 × 10−5, t-test). Middle and right: similar to left, for the subset of NREM episodes preceding wakefulness (P = 0.0089) and that preceding REM sleep (P < 10−5). The increase in firing rate was stronger for NREM episodes preceding REM sleep than those preceding wakefulness (P = 0.0003, paired t-test). e, Correlation between firing rate and EEG power in different frequency bands (delta, 0.5–4 Hz; theta, 4–12 Hz; sigma, 9–25 Hz; gamma, 40–120 Hz) during NREM sleep. The firing rate of each neuron was z-scored, and the power within each frequency band was normalized by its mean across each recording session. Firing rates and EEG power in each frequency band were discretized in 2.5 s bins. For each bin assigned to NREM sleep, we plotted the power in each frequency bands versus the corresponding firing rate. Linear regression was used to determine whether the power in each frequency band and the firing rate were positively or negatively correlated. The correlation was positive for theta (P < 10−5) and sigma (P < 10−5), negative for gamma (P < 10−5), and not significant for delta (P = 0.58).
Extended Data Figure 7 Mapping of monosynaptic inputs and axon projections of GABAPOA→TMN neurons and axon projections of POA CCK, CRH, TAC1 neurons.
a, Schematic of cTRIO to map monosynaptic inputs to GABAPOA→TMN (left) or GABAPOA→PFC (right) neurons. Mouse brain figure adapted with permission from ref. 31. Middle, coronal section of a mouse brain at the POA stained with Hoechst (blue). A region within the square is magnified in the inset. Arrowheads indicate starter cells (yellow) at the injection site (scale bar in inset, 50 μm). b, Optogenetic activation of GABAPOA→PFC neurons. Shown is the percentage of time in wake, NREM, or REM state before, during, and after laser stimulation (blue shading, 10 Hz, 120 s), averaged from six mice (P < 0.001 for wake and NREM, P = 0.003 for REM, bootstrap). Shading for each trace, 95% confidence interval. c, Average fractional inputs in cTRIOPOA→TMN (purple) or cTRIOPOA→PFC (grey) tracing (P = 0.0002 for hypothalamus, P = 0.02 for amygdala, P = 0.03 for striatum, P = 0.001 for midbrain, P = 0.003 for pons, t-test). N = 3 mice in each group. Error bar, ±s.e.m. d, Schematic of the axon projection mapping experiment. e, Coronal sections containing POA, TMN, and dorsomedial hypothalamus regions stained with Hoechst (blue). A region within the square is magnified in the inset. Red, synaptophysin–mRuby; green, mGFP. f, Projection levels (quantified by mGFP-labelled axonal arbors) in different brain areas normalized by that in the TMN. Shown are only areas with projections >10% of the TMN projection. Hb, habenula; LH, lateral hypothalamus. N = 3 mice. g–i, Axon projections of POA CCK, CRH, TAC1 neurons. Top: coronal section containing the TMN region. Red, immunostaining for HDC showing histaminergic neurons. Bottom: projection levels (quantified by eYFP-labelled axonal arbors) in different brain areas normalized by that in the TMN. N = 3, 3, 4 mice respectively.
Extended Data Figure 8 Identification of genetic markers for GABAPOA→TMN neurons using TRAP and single-cell RNA-seq, and overlap between each identified marker and GAD and between the markers in the POA.
a, TRAP; shown is bioanalyzer trace of immunoprecipitated RNA. FU, fluorescence units. b, Histogram display of differentially expressed genes (immunoprecipitation per input). c, Fragments per kilobase of transcript per million mapped reads (FPKM) immunoprecipitation versus FPKM input (log scale). Several marker genes enriched in GABAPOA→TMN neurons (Cck, Crh, Slc32a1, Rpl10a) are highlighted. Red dots, genes that are significantly different in immunoprecipitation versus input (P < 0.05, Fisher’s exact test); blue dots, non-significant genes. d, Single-cell RNA-seq; shown is heat map of expression levels of several cell-type markers (for example, Gad1, Gad2, Slc32a1, Slc17a6, and Chat) and all neuropeptide-encoding genes (on the basis of the list of ref. 43 plus GAL) in cholinergic neurons in the nucleus basalis and eYFP-labelled GABAPOA→TMN neurons in the POA. Tac1 and Pdyn are highly expressed in GABAPOA→TMN neurons. RPKM, reads per kilobase of transcript per million mapped reads. e–h, Overlap between each identified marker and GAD. A representative image showing overlap between CCK-ChR2–eYFP (e), CRH–eYFP (f), TAC1–eYFP (g), DYN-ChR2–eYFP (h), and FISH for mRNA encoding GAD1/2. Arrowheads indicate cells co-labelled with GAD1/2 probe and eYFP. Mouse brain figure adapted with permission from ref. 31. i–k, Overlap between the markers. A representative image showing overlap between CCK and CRH (i), CRH and TAC1 (j), or CCK and TAC1 (k) using double FISH for both peptides. Arrowheads indicate co-labelled cells. l, Percentage of cells expressing each peptide marker that are GAD1/2 positive (n = 2 or 3 mice per marker). m, Quantification of overlap between CCK and CRH, TAC1 and CRH, or TAC1 and CCK (n = 3 mice per pair). Error bar, ±s.e.m.
Extended Data Figure 9 Effect of laser activation of CCK, CRH, TAC1, and PDYN neurons on transition probability between each pair of brain states.
a, Transition probability within each 10 s period in CCK neuron activation experiment. Error bar, 95% confidence interval (bootstrap). N = 4 mice. b–d, Similar to a, for CRH, TAC1, and PDYN neuron activation. N = 5, 7, 5 mice, respectively.
Extended Data Figure 10 Pharmacogenetic inactivation of CCK, CRH, and TAC1 neurons, optogenetic inactivation of CCK neurons, optogenetic activation of GAL neurons, and optogenetic activation of PDYN neurons in the POA.
a, Pharmacogenetic inactivation of CCK neurons. Left, a representative image showing hM4D(Gi)–mCherry expression in the POA of a CCK-Cre mouse and an enlarged view of the region in white box. Mouse brain figure adapted with permission from ref. 31. Middle, effect of CNO injection in CCK-Cre mice expressing hM4D(Gi). Each bar shows the percentage of time in each brain state during the first 4 h of the recording session, after injection of vehicle (grey) or CNO (blue). Error bar, ±s.e.m. (n = 6 mice, P = 0.022, 0.025, 0.044 for REM, wake, and NREM, paired t-test). Right, effect of CNO injection in control CCK-Cre mice not expressing hM4D(Gi) (n = 4 mice, P = 0.27, 0.46, and 0.29 for REM, wake, and NREM, paired t-test). The effect of CNO was significantly different between hM4D(Gi)-expressing and control mice (P = 0.006, 0.036, and 0.014 for REM, wake, and NREM, t-test). b, Similar to a, for CRH neuron inactivation (n = 6 mice, P = 0.015, 0.018, 0.024). For control, n = 5 mice; P = 0.58, 0.41, and 0.12. Difference between hM4D(Gi) and control, P = 0.003, 0.03, and 0.014. c, For TAC1 neuron inactivation (n = 6 mice, P = 0.0057, 0.0026, 0.0095). For control, n = 4 mice; P = 0.92, 0.13, and 0.06. Difference between hM4D(Gi) and control, P = 0.001, 0.005, and 0.037. d, Optogenetic inhibition of POA CCK neurons suppresses sleep and enhances wakefulness. Shown is the percentage of time in wake, NREM, or REM state before, during, and after laser stimulation (blue shading, constant light, 60 s), averaged from four mice (P < 0.0001 for wake and NREM, P = 0.008 for REM, bootstrap). e, Similar to d, with laser stimulation of POA GAL neurons (blue shading, 10 Hz, 60 s), averaged from four mice (P < 0.0001 for increase in wakefulness, bootstrap). f, Similar to d, with optogenetic stimulation of POA PDYN neurons (blue shading, 10 Hz, 120 s), averaged from five mice (P = 0.42, 0.002, and 0.0003 for REM, NREM, and wake, bootstrap). Shading for each trace, 95% confidence interval.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-2. (PDF 4662 kb)
Optogenetic activation of GABAPOA→TMN neurons promotes sleep
2 laser stimulation trials, including 30 s before and after each laser stimulation period. The EEG spectrogram, EMG trace and color-coded hypnogram are shown on the right. Laser stimulation periods are depicted by the blue bar on the top right and additionally indicated as a blue square in the upper right corner of the movie frame. The video is shown at 8× the original speed. (MOV 1578 kb)
Optogenetic activation of GABAPOA neurons promotes wakefulness
2 laser stimulation trials, including 30 s before and 60 s after each laser stimulation period. The video is shown at 8× the original speed. (MOV 1838 kb)
Optogenetic activation of CCK neurons in the POA promotes sleep
2 laser stimulation trials, including 30 s before and after each laser stimulation period. The video is shown at 8× the original speed. (MOV 1627 kb)
Rights and permissions
About this article
Cite this article
Chung, S., Weber, F., Zhong, P. et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 545, 477–481 (2017). https://doi.org/10.1038/nature22350
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22350
This article is cited by
-
The dynamic state of a prefrontal–hypothalamic–midbrain circuit commands behavioral transitions
Nature Neuroscience (2024)
-
A midbrain GABAergic circuit constrains wakefulness in a mouse model of stress
Nature Communications (2024)
-
Activation of lateral preoptic neurons is associated with nest-building in male mice
Scientific Reports (2024)
-
The medial preoptic area mediates depressive-like behaviors induced by ovarian hormone withdrawal through distinct GABAergic projections
Nature Neuroscience (2023)
-
Neuro-orchestration of sleep and wakefulness
Nature Neuroscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.