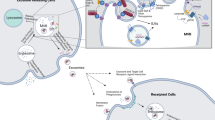
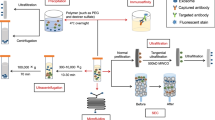
Abstract
The mutant form of the GTPase KRAS is a key driver of pancreatic cancer but remains a challenging therapeutic target. Exosomes are extracellular vesicles generated by all cells, and are naturally present in the blood. Here we show that enhanced retention of exosomes, compared to liposomes, in the circulation of mice is likely due to CD47-mediated protection of exosomes from phagocytosis by monocytes and macrophages. Exosomes derived from normal fibroblast-like mesenchymal cells were engineered to carry short interfering RNA or short hairpin RNA specific to oncogenic KrasG12D, a common mutation in pancreatic cancer. Compared to liposomes, the engineered exosomes (known as iExosomes) target oncogenic KRAS with an enhanced efficacy that is dependent on CD47, and is facilitated by macropinocytosis. Treatment with iExosomes suppressed cancer in multiple mouse models of pancreatic cancer and significantly increased overall survival. Our results demonstrate an approach for direct and specific targeting of oncogenic KRAS in tumours using iExosomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hidalgo, M. & Von Hoff, D. D. Translational therapeutic opportunities in ductal adenocarcinoma of the pancreas. Clin. Cancer Res. 18, 4249–4256 (2012)
Chang, D. K., Grimmond, S. M. & Biankin, A. V. Pancreatic cancer genomics. Curr. Opin. Genet. Dev. 24, 74–81 (2014)
Collins, M. A. et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Invest. 122, 639–653 (2012)
Collins, M. A. et al. Metastatic pancreatic cancer is dependent on oncogenic Kras in mice. PLoS One 7, e49707 (2012)
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012)
Gysin, S., Salt, M., Young, A. & McCormick, F. Therapeutic strategies for targeting Ras proteins. Genes Cancer 2, 359–372 (2011)
Pecot, C. V. et al. Therapeutic silencing of KRAS using systemically delivered siRNAs. Mol. Cancer Ther. 13, 2876–2885 (2014)
Yuan, T. L. et al. Development of siRNA payloads to target KRAS-mutant cancer. Cancer Discov. 4, 1182–1197 (2014)
Xue, W. et al. Small RNA combination therapy for lung cancer. Proc. Natl Acad. Sci. USA 111, E3553–E3561 (2014)
Réjiba, S., Wack, S., Aprahamian, M. & Hajri, A. K-ras oncogene silencing strategy reduces tumor growth and enhances gemcitabine chemotherapy efficacy for pancreatic cancer treatment. Cancer Sci. 98, 1128–1136 (2007)
Zorde Khvalevsky, E. et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20723–20728 (2013)
van der Meel, R. et al. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J. Control Release 195, 72–85 (2014)
Kowal, J., Tkach, M. & Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 29, 116–125 (2014)
Johnsen, K. B. et al. A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta 1846, 75–87 (2014)
Vader, P., Mol, E. A., Pasterkamp, G. & Schiffelers, R. M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 106, 148–156 (2016)
Kaur, S. et al. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol. 37, 49–59 (2014)
Kibria, G. et al. A rapid, automated surface protein profiling of single circulating exosomes in human blood. Sci. Rep. 6, 36502 (2016)
Brown, E. J. & Frazier, W. A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 11, 130–135 (2001)
Jaiswal, S. et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138, 271–285 (2009)
Chao, M. P., Weissman, I. L. & Majeti, R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 24, 225–232 (2012)
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013)
Simões, S. et al. Cationic liposomes for gene delivery. Expert Opin. Drug Deliv. 2, 237–254 (2005)
Willingham, S. B. et al. The CD47-signal regulatory protein alpha (SIRPα) interaction is a therapeutic target for human solid tumors. Proc. Natl Acad. Sci. USA 109, 6662–6667 (2012)
Nakase, I., Kobayashi, N. B., Takatani-Nakase, T. & Yoshida, T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci. Rep. 5, 10300 (2015)
Özdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014)
Ijichi, H. et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J. Clin. Invest. 121, 4106–4117 (2011)
Sherman, M. H. et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014)
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012)
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009)
Guerra, C. & Barbacid, M. Genetically engineered mouse models of pancreatic adenocarcinoma. Mol. Oncol. 7, 232–247 (2013)
Ying, H. et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 30, 355–385 (2016)
Gidekel Friedlander, S. Y. et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 16, 379–389 (2009)
Pylayeva-Gupta, Y., Lee, K. E., Hajdu, C. H., Miller, G. & Bar-Sagi, D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21, 836–847 (2012)
Biankin, A. V. et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491, 399–405 (2012)
Eser, S., Schnieke, A., Schneider, G. & Saur, D. Oncogenic KRAS signalling in pancreatic cancer. Br. J. Cancer 111, 817–822 (2014)
Singh, H., Longo, D. L. & Chabner, B. A. Improving prospects for targeting RAS. J. Clin. Oncol. 33, 3650–3659 (2015)
Zeitouni, D., Pylayeva-Gupta, Y., Der, C. J. & Bryant, K. L. KRAS mutant pancreatic cancer: no lone path to an effective treatment. Cancers (Basel) 8, E45 (2016)
Golan, T. et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 6, 24560–24570 (2015)
Kordelas, L. et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28, 970–973 (2014)
Clayton, A., Harris, C. L., Court, J., Mason, M. D. & Morgan, B. P. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol. 33, 522–531 (2003)
Gomes-da-Silva, L. C. et al. Lipid-based nanoparticles for siRNA delivery in cancer therapy: paradigms and challenges. Acc. Chem. Res. 45, 1163–1171 (2012)
Zheng, X. et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530 (2015)
Melo, S. A. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182 (2015)
Melo, S. A. et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 26, 707–721 (2014)
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011)
El-Andaloussi, S. et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protocols 7, 2112–2126 (2012)
Thery, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter 3, Unit 3.22 (2006)
Ma, J. B., Ye, K. & Patel, D. J. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature 429, 318–322 (2004)
Du, Q., Thonberg, H., Wang, J., Wahlestedt, C. & Liang, Z. A systematic analysis of the silencing effects of an active siRNA at all single-nucleotide mismatched target sites. Nucleic Acids Res. 33, 1671–1677 (2005)
Rachagani, S. et al. Activated KrasG12D is associated with invasion and metastasis of pancreatic cancer cells through inhibition of E-cadherin. Br. J. Cancer 104, 1038–1048 (2011)
Poliseno, L. et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465, 1033–1038 (2010)
Acknowledgements
This work was primarily supported by the Cancer Prevention and Research Institute of Texas, the Knowledge Gap funding of MD Anderson Cancer Center, and NCI grant CA213233. Other support include: V.S.L. laboratory: UT MDACC Khalifa Bin Zayed Al Nahya Foundation; High Resolution Electron Microscopy Facility: Institutional Core Grant CA16672; MDACC Flow Cytometry core facility; and J.J.L. laboratory: NIH P30CA16672; MDACC Small Animal Imaging Facility: NIH P30-CA016672 and 5U24-CA126577. The mass spectrometry-related analysis (SAM) was supported by the NORTE-01-0145-FEDER-000029 (NORTE 2020; ERDF) and FEDER funds (POCI-01/0145-FEDER-016618) and FCT-PTDC/BIM-ONC/2754/2014. We thank K. Dunner Jr for the help with the transmission electron microscopy and immunogold techniques, E. Chang for slide scanning, D. Lundy for tissue processing, J. Carstens and C. Kahlert for independent analyses of tissue histopathology, J. Kaye and L. Gibson for mouse husbandry support, C. Kingsley and D. Lundy for MRI support, E. Lawson for IVIS imaging help, and P. Correa-de-Sampaio for microscopy imaging help.
Author information
Authors and Affiliations
Contributions
R.K. conceptually designed the experimental strategy, provided intellectual input and helped to write the manuscript. H.S. and V.S.L. injected mice orthotopically with tumour cells. H.S. performed necropsy analyses and quantification of exosomes in pancreas tissue sections. V.S.L. provided intellectual input, extracted RNA and quantified siRNA loading in exosomes by qPCR analyses, stained tissues for Kras, helped with in vivo experiments, designed the experimental strategy, prepared figures and wrote the manuscript. S.K. and S.Y. prepared exosomes (cultured cells for exosome collection and preparation by ultracentrifugation), generated iExosomes (electroporation and wash steps involved in the generation of iExosome and NanoSight measurements), and treated mice with iExosomes. S.Y. also performed an independent analysis of in vivo imaging data, generated and genotyped KTC and KPC mice for iExosome treatment, and helped prepare the figures. S.K. provided intellectual input, helped design the experimental strategy, and performed experiments, including treatment of mice with iExosomes, preparation of exosomes for sucrose gradient and qPCR analyses. Unless otherwise noted, S.K. performed the independent replication of the experiments. S.K. performed sucrose gradient analyses, NanoSight measurements, flow cytometry experiments and analyses, immunostaining and analyses, RNA extraction and qPCR of treated cells, MTT and TUNEL assays, macropinocytosis and western blot analyses, analysed data, prepared figures, and helped to write the manuscript. S.A.M. advised and provided intellectual input to the siRNA identification experiments, MTT assays, qPCR analyses, western blots for p-ERK, and one PANC-1 tumour study and one KTC in vivo experiment. C.F.R. provided the mass spectrometry data and the relevant section in the Methods. J.J.L. reviewed the source data, advised on experimental design, and supervised statistical analyses.
Corresponding author
Ethics declarations
Competing interests
MD Anderson Cancer Center and R.K. hold patents in the area of exosome biology and are licensed to Codiak Biosciences Inc. MD Anderson Cancer Center and R.K. are stock equity holders in Codiak Biosciences Inc. R.K. receives research support from Codiak Biosciences Inc. and serves as a member of the board of directors. V.S.L. served once as a paid consultant for Codiak Biosciences Inc.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Exosomes purification and siRNA loading.
a, Exosome and liposome numbers and size distribution using NanoSight analysis. b, Transmission electron micrographs of exosomes stained with secondary antibody only (left) or stained for CD9 by immunogold (right). Scale bars, 100 nm. c, Flow cytometry analyses of CD63 and CD47 on exosomes. n = 3 distinct exosome isolations. d, Flow cytometry analyses and quantification of exosomal proteins CD63 and CD47 in liposomes. e, Schematic representation of electroporation of RNAi into exosomes. f, Schematic (left) and fluorescence intensity plots (right and bottom) of sucrose gradient layers (from the ‘bottom-up’ method). UC, ultracentrifuge. Results from three independent experiments are shown. g, Schematic (left) and fluorescence intensity plots (right and bottom) of sucrose gradient layers (from the ‘top-down’ method). Results from three independent experiments are shown. Data are mean ± s.e.m. See accompanying Source Data.
Extended Data Figure 2 Tissue distribution and clearance of iExosomes.
a, Flow cytometry analyses (left) and quantification (right) of the comparison of the binding efficiency to aldehyde sulfate beads. n = 3 distinct batches of exosomes and liposomes. b, Flow cytometry analyses (left) and quantification (right) of exosomes and liposomes electroporated with AF647-tagged siRNA, isolated from the plasma of C57BL/6 and nude (nu/nu) mice, 24 h after injection. n = 3 mice per group. c, Flow cytometry analyses (from data in Fig. 1b) of exosomes with AF647-tagged siRNA in the circulation of mice. d, Representative micrographs of the indicated organs of mice injected intraperitoneally with PKH67-labelled BJ fibroblast exosomes. n = 3 mice. Quantification is shown in Fig. 1c. e, f, Flow cytometry analyses of pancreas cells 6 h (e; quantification in Fig. 1d) and 24 h (f) after injection of siKrasG12D exosomes. Data are mean ± s.e.m. ****P < 0.0001, one-way ANOVA. See accompanying Source Data.
Extended Data Figure 3 CD47-induced monocyte clearance and iExosome characterization.
a, Schematic representation of gating strategy for data in Fig. 1e. b, Flow cytometry analysis of CD11b+ cells in the circulation (untreated, n = 4 mice), liposomes (n = 7 mice) and exosomes (n = 7 mice). Unstained sample was used to control for background signal. c, Representative dot plots from Fig. 1e. d, Flow cytometry analyses of SIRPα expression from AF647+CD11b+ monocytes. e, Flow cytometry analyses of the binding efficiency of CD47-neutralizing antibodies to exosomes. n = 3 distinct batches of exosomes. f, Quantification of the number of exosomes per millilitre in the plasma of wild-type C57BL/6 mice (n = 5) versus CD47-knockout mice (n = 7). Data are mean ± s.e.m. *P < 0.05, ***P < 0.001, one-way ANOVA (b, e) and unpaired two-tailed t-test (f). See accompanying Source Data.
Extended Data Figure 4 iExosomes specifically target KRASG12D expression.
a, KRASG12D transcript levels in PANC-1 cells. n = 3 independent experiments. b, c, 1/Ct values from qPCR analysis under the listed conditions, to determine the loading efficiency of siRNA. Standards (siKrasG12D, 1:2 and 1:4 dilution): n = 1; experimental groups: n = 3 independent experiments. d, KRASG12D transcript levels in PANC-1 cells. n = 3 independent experiments. The experiments with 400 exosomes per cell are the same data shown in a. e–g, KRASG12D transcript levels in PANC-1 cells under the listed conditions. In all groups, n = 3 independent experiments. h, Western blotting in PANC-1 cells for p-ERK and vinculin. n = 2 independent experiments. i, RAS pull-down assay. j, k, PANC-1 cells MTT assay (n = 5 partitions of indicated treatments with 3 or 6 wells for each partition of treatment) (j) and a separate independent experiment (k). l, m, TUNEL assay (n = 3 distinct wells of PANC-1 cells) (l) and a separate independent experiment (m). n, Flow cytometry analysis of apoptosis in PANC-1 cells. Three different treatments were used to treat n = 3 distinct wells of cells. o, Wild-type KRAS transcript levels in BxPC-3 cells. n = 3 independent experiments. p, KRASG12V transcript levels in Capan-1 cells. n = 3 independent experiments. q, KRASG12C transcript levels in MIA PaCa-2 cells. n = 3 independent experiments. r, s, MTT assay in BxPC-3 cells (n = 5 partitions of treatment given to 3 wells each) (r) and a separate independent experiment (s). t, MTT assay in Capan-1 cells (n = 3 partitions of treatment given to 10 wells each). u, MTT assay in MIA PaCa-2 cells (n = 3 partitions of treatment given to 10 wells each). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, one-way ANOVA (for all, except h, iLipos), and two-tailed t-test (h, iLipos). See accompanying Source Data. For uncropped blots in h and i, see Supplementary Fig. 1.
Extended Data Figure 5 KrasG12D RNAi-containing exosomes suppress PANC-1 but not BxPC-3 orthotopic tumour growth.
a, Experimental scheme. b, Representative micrographs depicting accumulation of internalized AF647-tagged siRNA from exosomes. Scale bar, 100 μm. c, PANC-1 orthotopic tumour growth (an inset from data in Fig. 2c). n = 6 mice (PBS, control Exo); n = 3 mice (siKrasG12D iLipo, shKrasG12D iLipo); n = 7 mice (siKrasG12D iExo, shKrasG12D iExo); n = 5 mice (siScrbl iExo, shScramble iExo). Treatment groups were compared to PBS control at day 42 after cancer cell injection, or day 28 for siKrasG12D exosomes group. d, Tumour growth (bioluminescence) at day 77 (total flux). n = 4 mice (PBS, siScramble iExo); n = 3 mice (control Exo, shKrasG12D iLipo, shScramble iExo); n = 6 mice (shKrasG12D iExo). e, Luciferase activity at days 7, 35 and 77, and moribund stage or day 200 (shKrasG12D iExo) after cancer cell injection. Some of these panels are also shown in Fig. 2a. f, PANC-1 orthotopic tumour growth (bioluminescence) over time (total flux). n = 7 mice (PBS, shKrasG12D iExo); n = 6 mice (control Exo); n = 4 mice (shKrasG12D iLipo); n = 5 mice (shScramble iExo, siScramble iExo). g, Representative H&E staining of the PANC-1 orthotopic tumours. Scale bars, 100 μm. h, Representative micrographs (left) and quantification (right) of tumours immunostained for p-AKT. Scale bars, 100 μm. n = 4 mice (control Exo); n = 6 mice (shKrasG12D iExo). i, j, BxPC-3 orthotopic tumour growth (bioluminescence) over time. Radiance (i) or total flux (j). n = 3 mice per group. k, Luciferase activity at days 14 and 77 after cancer cell (BxPC-3) injection. l, Representative H&E staining of the BxPC-3 orthotopic tumours at the indicated experimental end points. Scale bars, 100 μm. m, Kaplan–Meier survival curve of BxPC-3 tumour-bearing mice. n = 3 mice per group. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, one-way ANOVA (d, f, i, j), unpaired two-tailed t-test (c, h) and log-rank Mantel–Cox test (m). See accompanying Source Data.
Extended Data Figure 6 Anti-tumour response of iExosomes in orthotopic models.
a, Flow cytometry analyses and quantification of CD81 in exosomes under listed conditions. n = 3 independent experiments. b, PANC-1 orthotopic tumour growth (bioluminescence) over time. n = 6 mice per group. c, Kaplan–Meier survival curve of PANC-1 tumour-bearing mice. n = 6 mice per group. d, Tumour growth (bioluminescence) at day 45. n = 6 mice per group. e–j, PANC-1 orthotopic tumour growth (bioluminescence) over time, depicting separate groups from b (e, g, i) and Kaplan–Meier survival curve, depicting the separate groups from c (f, h, i). n = 6 mice per group. k, Tumour growth (bioluminescence) at day 42. n = 3 mice per group. Experimental groups compared to PBS control. l, Growth of PANC-1 orthotopic tumours (bioluminescence) over time (total flux). n = 3 mice per group. m, Luciferase activity at days 10 and 42 after cancer cell (PANC-1) injection. n, Surface lung nodules of KPC689 mice. n = 8 mice per group. o, Tumour weights. n = 8 mice per group. siKrasG12D iExo group is compared to other treatment groups. p, Bioluminescent KPC689 orthotopic tumours in nu/nu mice. n = 8 mice per group. q, Tumour weights. n = 8 mice per group. r, Kaplan–Meier curve of KPC689 nu/nu mice. n = 8 in each group. Data are mean ± s.e.m. **P < 0.01, ***P < 0.001, ****P < 0.0001, unpaired two-tailed t-test (a, q), ANOVA (d, k, n, o) and log-rank Mantel–Cox test (c, e–j, r). See accompanying Source Data.
Extended Data Figure 7 Pancreas localization and macropinocytosis promotes iExosome uptake into tumour cells.
a, Representative images (top) and quantification (bottom) of pancreas structure in KTC mice injected with exosomes containing AF647-tagged siRNA. n = 3 mice. b, Representative images (left) and quantification (right) of pancreas of mice injected with the indicated conditions. n = 3 mice per grouph. c, Representative images for data in Fig. 3e–h. d, Quantification of macropinocytic and exosomes uptake (independent experiment, identical statistical analyses). e, Uptake of exosomes or liposomes containing AF647-tagged siRNA into PANC-1 cells. n = 3 independent experiments. f, Uptake of CM-DiI-tagged CD47-knockout versus wild-type exosomes in PANC-1 cells. n = 3 independent experiments. g, Uptake of CM-DiI-tagged CD47-knockout versus wild-type exosomes in BxPC-3 cells. n = 3 independent experiments. Scale bars, 100 μm (a, b, e, f) and 50 μm (c). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, one-way ANOVA (all except b) and unpaired two-tailed t-test (b). See accompanying Source Data.
Extended Data Figure 8 Treatment of KTC genetically engineered mice with iExosomes.
a, Experimental scheme. b, Accumulation of internalized AF647-tagged siRNA from exosomes. c, Kaplan–Meier survival curve of KTC mice. n = 8 mice (PBS); n = 5 mice (gemcitabine). d, Tumour burden at the experimental end point. n = 7 mice (control Exo, siKrasG12D iExo); n = 5 mice (shKrasG12D iExo). e, H&E-stained tumours from KTC mice (left), and relative percentages in histological phenotypes (right). n = 4 mice per group. f, g, Kaplan–Meier curve of KTC mice (f) and percentage tumour burden (g). n = 3 mice per group. h, Representative micrographs of tumours immunostained for p-AKT, α-SMA and Kras from 44-day-old KTC mice in the indicated experimental groups. n = 3 mice per group. Scale bars, 100 μm (b, e, h). *P < 0.05, **P < 0.01, ****P < 0.0001, one-way ANOVA (d), unpaired two-tailed t-test (e, g, h) and log-rank Mantel–Cox test (f). See accompanying Source Data.
Extended Data Figure 9 Evaluation of the cytotoxicity and off-target effect of iExosomes.
a, Change in the percentage of mouse body weights, before and after treatment, in the listed groups and cohorts. One-way ANOVA (related to Figs 2c, 4a) and unpaired two-tailed t-test comparing the indicated groups at pre-treatment, day 40, and the experimental end point (related to Figs 3c, 4b, i). In all cases, there were no statistical differences observed. b, Mouse toxicity tests, consisting of blood urea nitrogen (BUN, aspartate transaminase (AST) and alanine transaminase (ALT) in the listed groups. One-way ANOVA comparing KTC mice, unpaired two-tailed t-test comparing nude mice, and unpaired two-tailed t-test comparing 689KPC mice. In all cases, there were no statistical differences observed. c, H&E and Kras immunostaining of the listed organs in KTC (early treatment) mice. n = 3–5 mice evaluated per organ. One-way ANOVA comparing the indicated groups in each organ. In all cases, there were no statistical differences observed. Data are mean ± s.e.m. See accompanying Source Data.
Extended Data Figure 10 iExosomes suppress pancreatic cancer progression in the KPC orthotopic mouse model.
a, b, MRI of KPC orthotopic tumours (n = 9 mice per group) (a) and each individual tumour (b). c, Tumour volume as measured by MRI. n = 9 mice per group. d, Representative axial images. e, Tumour weight at the experimental end point. n = 9 mice (siScrbl iExo); n = 8 mice (siKrasG12D iExo). f, Change in the percentage of mouse body weights, before and after treatment (end point). n = 9 mice per group. g, Representative gross images of two KPC orthotopic mice that died on day 16 post-treatment start (PTS) (siScrbl iExo) or was euthanized on day 16 PTS (siKrasG12D iExo). h, KrasG12D transcript levels in KPC689 cells. n = 3 independent experiments. i, Kaplan–Meier survival curve of KPC orthotopic tumour-bearing mice. n = 9 mice (siScrbl iExo); n = 8 mice (siKrasG12D iExo). j, Macroscopic metastatic nodules. n = 9 mice per group. k, H&E-stained tissues. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, unpaired two-tailed t-test (a, e, h), one-way ANOVA (c), and log-rank Mantel–Cox test (i). See accompanying Source Data.
Supplementary information
Supplementary Information
This file contains Supplementary Text regarding the Results sections, a Supplementary Discussion, Supplementary details regarding the figure legends, Supplementary References and Supplementary Figure 1, the uncropped blots. (PDF 2308 kb)
Supplementary Table 1
This file contains results from Mass Spectrometry analysis of exosomes from T3M4 exosomes from CD24+CD44+ cells, see the Methods section for details. (XLSX 88 kb)
Source data
Rights and permissions
About this article
Cite this article
Kamerkar, S., LeBleu, V., Sugimoto, H. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017). https://doi.org/10.1038/nature22341
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22341
This article is cited by
-
Exosomal mediators in sepsis and inflammatory organ injury: unraveling the role of exosomes in intercellular crosstalk and organ dysfunction
Military Medical Research (2024)
-
Extracellular vesicle encapsulated nicotinamide delivered via a trans-scleral route provides retinal ganglion cell neuroprotection
Acta Neuropathologica Communications (2024)
-
The remodeling of ovarian function: targeted delivery strategies for mesenchymal stem cells and their derived extracellular vesicles
Stem Cell Research & Therapy (2024)
-
An exosomal strategy for targeting cancer-associated fibroblasts mediated tumors desmoplastic microenvironments
Journal of Nanobiotechnology (2024)
-
Current progression in application of extracellular vesicles in central nervous system diseases
European Journal of Medical Research (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.