Abstract
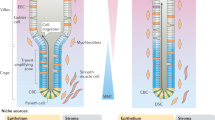
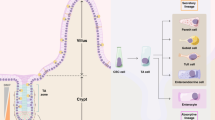
The small intestinal epithelium self-renews every four or five days. Intestinal stem cells (Lgr5+ crypt base columnar cells (CBCs)) sustain this renewal and reside between terminally differentiated Paneth cells at the bottom of the intestinal crypt1. Whereas the signalling requirements for maintaining stem cell function and crypt homeostasis have been well studied, little is known about how metabolism contributes to epithelial homeostasis. Here we show that freshly isolated Lgr5+ CBCs and Paneth cells from the mouse small intestine display different metabolic programs. Compared to Paneth cells, Lgr5+ CBCs display high mitochondrial activity. Inhibition of mitochondrial activity in Lgr5+ CBCs or inhibition of glycolysis in Paneth cells strongly affects stem cell function, as indicated by impaired organoid formation. In addition, Paneth cells support stem cell function by providing lactate to sustain the enhanced mitochondrial oxidative phosphorylation in the Lgr5+ CBCs. Mechanistically, we show that oxidative phosphorylation stimulates p38 MAPK activation by mitochondrial reactive oxygen species signalling, thereby establishing the mature crypt phenotype. Together, our results reveal a critical role for the metabolic identity of Lgr5+ CBCs and Paneth cells in supporting optimal stem cell function, and we identify mitochondria and reactive oxygen species signalling as a driving force of cellular differentiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5 . Nature 449, 1003–1007 (2007)
Zhang, J. et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 30, 4860–4873 (2011)
Owusu-Ansah, E. & Banerjee, U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461, 537–541 (2009)
Tormos, K. V. et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 14, 537–544 (2011)
Hamanaka, R. B. et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci. Signal. 6, ra8 (2013)
Yilmaz, Ö. H. et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490–495 (2012)
Yin, X. et al. Engineering stem cell organoids. Cell Stem Cell 18, 25–38 (2016)
Barker, N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19–33 (2014)
Yin, X. et al. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat. Methods 11, 106–112 (2014)
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011)
Mustata, R. C. et al. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Reports 5, 421–432 (2013)
Fordham, R. P. et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13, 734–744 (2013)
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013)
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011)
Kankotia, S. & Stacpoole, P. W. Dichloroacetate and cancer: new home for an orphan drug? Biochim. Biophys. Acta 1846, 617–629 (2014)
Michelakis, E. D., Webster, L. & Mackey, J. R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer 99, 989–994 (2008)
Zhdanov, A. V., Waters, A. H., Golubeva, A. V., Dmitriev, R. I. & Papkovsky, D. B. Availability of the key metabolic substrates dictates the respiratory response of cancer cells to the mitochondrial uncoupling. Biochim. Biophys. Acta 1837, 51–62 (2014)
Aguer, C. et al. Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One 6, e28536 (2011)
Gutscher, M. et al. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 5, 553–559 (2008)
Tormos, A. M., Taléns-Visconti, R., Nebreda, A. R. & Sastre, J. p38 MAPK: a dual role in hepatocyte proliferation through reactive oxygen species. Free Radic. Res. 47, 905–916 (2013)
Wagner, E. F. & Nebreda, A. R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549 (2009)
Regot, S., Hughey, J. J., Bajar, B. T., Carrasco, S. & Covert, M. W. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 157, 1724–1734 (2014)
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009)
Schewe, M. et al. Secreted phospholipases A2 are intestinal stem cell niche factors with distinct roles in homeostasis, inflammation, and cancer. Cell Stem Cell 19, 38–51 (2016)
Merlos-Suárez, A. et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 8, 511–524 (2011)
Otsuka, M. et al. Distinct effects of p38α deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology 138, 1255–1265 (2010)
Liang, R. & Ghaffari, S. Stem cells, redox signaling, and stem cell aging. Antioxid. Redox Signal. 20, 1902–1916 (2014)
Hoffmann, S., Spitkovsky, D., Radicella, J. P., Epe, B. & Wiesner, R. J. Reactive oxygen species derived from the mitochondrial respiratory chain are not responsible for the basal levels of oxidative base modifications observed in nuclear DNA of Mammalian cells. Free Radic. Biol. Med. 36, 765–773 (2004)
Cleaver, J. E. et al. Mitochondrial reactive oxygen species are scavenged by Cockayne syndrome B protein in human fibroblasts without nuclear DNA damage. Proc. Natl Acad. Sci. USA 111, 13487–13492 (2014)
Song, I. S. et al. FOXM1-induced PRX3 regulates stemness and survival of colon cancer cells via maintenance of mitochondrial function. Gastroenterology 149, 1006–1016 (2015)
Van Lidth de Jeude, J. F., Vermeulen, J. L., Montenegro-Miranda, P. S., Van den Brink, G. R. & Heijmans, J. A protocol for lentiviral transduction and downstream analysis of intestinal organoids. J. Vis. Exp. http://dx.doi.org/10.3791/52531 (2015)
Baker, K. et al. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 284, 215–221 (1998)
Morgan, B., Sobotta, M. C. & Dick, T. P. Measuring E GSH and H2O2 with roGFP2-based redox probes. Free Radic. Biol. Med. 51, 1943–1951 (2011)
Chambers, M. C., Song, K. H. & Schneider, D. S. Listeria monocytogenes infection causes metabolic shifts in Drosophila melanogaster . PLoS One 7, e50679 (2012)
Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R. & Siuzdak, G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78, 779–787 (2006)
Xia, J., Sinelnikov, I. V., Han, B. & Wishart, D. S. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 43, W251–W257 (2015)
Acknowledgements
This work was financially supported by CGC.nl (M.J.R.-C., H.J.S.), Utrecht Life Sciences (M.M.), Dutch Cancer Society ((KWF), EMCR 2012-5473 (M.S.), and UU 2013-6070 (K.C.O.)) and from the Netherlands Institute of Regenerative Medicine (R.F.). We thank H. Bos, T. Dansen and S. van Mil for helpful discussions and proofreading; F. de Sauvage (Genentech) for providing DTR–LGR5–GFP mice; T. Dick (DKFZ) for mtGrx1–roGFP and I. Verlaan (UMC Utrecht) for R-spondin/Wnt3a-conditioned medium.
Author information
Authors and Affiliations
Contributions
M.J.R.-C. and B.M.T.B. conceived the project, designed and performed experiments and wrote the manuscript; M.S. designed and performed organoid reconstitution experiments; M.M. performed experiments; E.S. and J.G. performed metabolic measurements. M.P.-R. and N.V.-D. performed metabolic data analysis. A.S. performed FACS of intestinal cells. M.H. performed p38 IHC. K.C.O. and H.J.S. provided organoid cultures, and H.J.S. co-wrote the manuscript. R.F. designed experiments and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks T. Sato, A. Schulze and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Metabolic compartmentalization and mitochondria and redox state in the crypt.
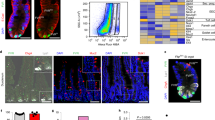
a, Top 10 most different metabolites between Lgr5+ CBCs, Paneth cells (PCs) and all other differentiated cell types (‘Neg.’). P value and false discovery rate (FDR) refer to the significance, and post hoc tests indicate the groups being compared. Results were obtained by analysis with http://metaboanalyst.ca. b, Live confocal microscopy of organoid crypts. All mitochondria (green) and high MMP (red) was determined by JC-1 staining. MMP/mitochondria ratio is represented in fire intensity scale (ImageJ). c, Live imaging of Lgr5–GFP-derived organoid stained with Mitotracker Deep-Red confirmed increased mitochondria in Lgr5+ CBCs. In both cases Hoechst was used to stain nuclei. Asterisks indicate stem cells in between Paneth cells (n = 1). d, Representative images of DTR–Lgr5–eGFP organoids grown in ENR medium or ENR medium supplemented with CHIR99021 (3 μM) and valproic acid (2 mM) during 72 h. e, Mitochondrial respiration/glycolysis (basal) was measured during mitochondrial stress test using Seahorse technology. Organoids were grown in ENR medium and after 24 h CHIR99021 and valproic acid were added to half of the plate during 72 h before running the assay. Graph represents mean and s.d. of 6 independent Seahorse experiments. Two-tailed t-test, *P < 0.05. f, ROS in the crypt was stained with CellRox (cytosolic ROS), MitoSOX (mitochondrial superoxide) and Mitotracker (mitochondria). Dashed yellow regions indicate Paneth cells. g, Quantification of ROS in CBCs (16) and Paneth cells (6) from 3 organoids. One representative experiment of 2 independent ones. Graphs show mean and s.d. Two tailed t-test, ****P < 0.0001, NS, not significant.
Extended Data Figure 2 WENR organoids represent a homogeneous population of glycolytic dividing cells that retain pluripotency.
a, Confocal images of organoid crypts. PC and nuclei were detected using WGA or Lyz antibody, and Hoechst, respectively. z-stacks are represented as 3D projections. b, Confocal live imaging of DTR–Lgr5–eGFP-derived mouse organoids. Lgr5+ cells are visualized in green. Dashed yellow region indicates a bud structure that constitutes a starting crypt. c, Confocal microscopy of immunostaining of an emerging crypt. Phalloidin was used to stain F-actin (in red) (n = 3 (a–c)). d, Gene expression of glycolytic genes in WENR organoids normalized by the expression in ENR organoids (dashed line) (n = 3). e, Bioenergetics was determined by Seahorse technology. Results show one representative experiment of 3 independent ones. f, Pyruvate/lactate ratio was analysed by DIMS metabolomics. Boxes and error bars correspond to mean and s.d. of 3 technical replicates of 3 biological samples. g, DCA inhibition of glycolysis in ENR or WENR grown organoids was determined by Seahorse technology 1 representative experiment of n = 3. h, Proliferation was measured by Ki67 gene expression by qPCR. i, Gene expression of PC markers (n = 5 independent experiments). j, Representative images of a differentiation assay and the effect of DCA and azide; spherical organoids (grown in WENR) forming crypts (WENR→ENR) and cryptic organoids (grown in ENR medium). k, Effect of glycolysis inhibition on crypt formation was analysed by differentiation assay using DCA or 2-deoxyglucose (one representative experiment, n = 2). l, Increased crypt formation by activation of mitochondria (h) was analysed by differentiation assay replacing glucose by galactose in ENR medium. Two-tailed t-test, ***P < 0.001; **P < 0.01; *P < 0.05.
Extended Data Figure 3 Mitochondrial OXPHOS and ROS signalling drive differentiation and crypt formation.
a, Mitochondrial DNA copy number was quantified by qPCR on total DNA. Plot represents one representative experiment (n = 2). b, Mitochondrial membrane potential and total mitochondria were analysed with the combination of Mitotracker Deep Red (mitochondria) and TMRM (mitochondrial membrane potential). Staining is represented as a ratio using fire intensity scale (ImageJ). Dashed yellow region indicates a bud structure that constitutes a starting crypt. c, Decreased crypt formation by inhibition of mitochondria was analysed by differentiation assay adding OXPHOS inhibitors: azide, myxothiazol (myxo.), rotenone and oligomycin (oligo.) (concentrations in μM). d, Toxicity of OXPHOS inhibitors was measured by counting the number of organoids growing in the depicted conditions. Values are plotted as percentages of the WENR condition (mean). e, Mitochondrial inhibition enhances inhibition of crypt formation occurring when plating ENR organoids in WENR medium. Azide (50 μM) was added to the medium, pictures were taken after 48 h and the number of crypts per organoid was counted using ImageJ. Average and s.d. are shown. Representative of one experiment (n = 2). f, The sensitivity to redox changes of the mtGrx1–roGFP in organoids was analysed by live imaging, applying 500 μM hydrogen peroxide and following the redox response in time. Images represent the ratio of oxidized/reduced sensor. g, Mitochondria redox state of a forming crypt was assessed by live imaging of mtGrx1–roGFP. h, i, Effect of ROS scavengers on crypt formation was analysed by differentiation assay (h) and Paneth cell markers gene expression (i) in the presence of EUK134 (5 μM) and mitoTEMPO (0.5 mM). j, k, The effect of triggering mitochondrial ROS on crypt formation was performed by differentiation assays applying paraquat and counting the number of crypts per organoids (j) and by the expression of Paneth cell markers by qPCR (k). One representative experiment of n = 3 (c, d) or n = 5 (h, j). Mean and s.d. are shown. Two tailed t-test; asterisks or NS indicate comparison to WENR→ENR control, * P < 0.05, ** P < 0.01.
Extended Data Figure 4 p38 activity drives differentiation and crypt formation in a mitochondrial ROS signalling dependent manner.
a, b, P38 activation was analysed by western blot and quantified. For gel source data see Supplementary Fig. 1. Organoids were treated with mitoTEMPO (0.5 mM overnight) or paraquat (7.5 mM, 1.5 h) (n = 3) (b). c, p38KTRClover organoids were challenged with anisomycin (75 μg ml−1) in order to analyse p38 activity. Images were obtained during 45 min after the addition of the compound. z-stacks of p38KTRClover or Hoechst/p38KTRClover ratio are represented as 3D projections and surface plot of 3D projections. Ratios are represented in either fire or real glow scale (ImageJ). d, Immunohistochemistry of p38 on mouse intestinal sections. e, f, Decreased differentiation and crypt formation upon p38 inhibition was analysed by differentiation assays (one representative experiment of n = 5) (e) and gene expression of Lyz1 and Lyz2 (f) in the presence of p38 inhibitors SB203580 and PH-797804. Mean and s.d. are shown (n = 5). Asterisks indicate comparison to WENR→ENR control; two-tailed paired t-test, *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Extended Data Figure 5 Mitochondrial activity, mitochondria ROS signalling and p38 activity regulate stem cell function of Lgr5+ CBCs.
a, The effects of the indicated compounds on respiration and glycolysis were tested after 3 h of the treatments using mitochondrial stress test (Seahorse technology). Bars and error bars represent the mean of the basal ratios and s.e.m. of 4 independent experiments with 4 technical replicates each. b, Cell viability after mitochondria and glycolysis inhibition treatments on CBCs and Paneth cells. Primary intestinal Lgr5+ CBCs and Paneth cells were treated with the indicated compounds for 2 h and then washed and stained with PI before FACS. PI negative and positive cells were counted as alive and dead, respectively. Values are plotted as percentages of the untreated cells (100%) indicated as a dashed line. Mean and s.d. of 2 independent experiments with 2 technical replicates each are represented in the graph. c, Effect of lactate and inhibition of pyruvate mitochondrial transport on mitochondrial respiration. Maximal respiration was measured by performing mitochondrial stress test after two hours of incubation with glucose or lactate with or without UK5099. Mean and s.d. are shown of 3 independent experiments with 4 technical replicates in each. d, Effect of lactate and inhibition of pyruvate mitochondrial transport on mitochondrial superoxide production. Mitochondrial superoxide was measured by FACS analysis of single cells stained with MitoSOX after 2 h of incubation with the indicated compounds. The graph represents the mean and s.d. of 4 independent experiments. e, P38 activity was monitored in p38KTRClover organoids growing for three days in the indicated conditions. P38 activity is measured by the ratio of cells with active (cytosolic localization)/inactive (nuclear localization) of the signal. The graph shows the counting of one representative experiment of 3 independent ones and each dot represents one organoid. f, The effect of inhibition of pyruvate transport to mitochondria on stem cell function was measured as number of reconstituted organoids from primary Lgr5+ CBCs in the depicted conditions. The graph represent the mean and s.d. of 3 independent experiments. Two-tailed t-test, *P < 0.05, **P < 0.01, ***P < 0.001. g, Number of crypts per reconstituted organoid and representative images of the referred conditions. The plot represents mean and s.e.m. of 35 organoids of one representative experiment of n = 6. Mann–Whitney test, ****P < 0.0001. In a, c–e, organoids were grown on ENR, CHIR99021 and valproic acid to enrich them with stem cells and the experiments were performed on single cells after trypsinization. In b, f, g, experiments were performed in mouse intestinal crypt FACS-sorted cells.
Supplementary information
Supplementary Figure 1
This file contains full scan images of western blots used for Extended Data 4a and b. Molecular weight markers are indicated; pp38 (phospho-p38) and p38. Dashed lines indicated cropped areas shown in Extended Data 4a and b. (PDF 261 kb)
Source data
Rights and permissions
About this article
Cite this article
Rodríguez-Colman, M., Schewe, M., Meerlo, M. et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543, 424–427 (2017). https://doi.org/10.1038/nature21673
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21673
This article is cited by
-
Hypoxic preconditioning accelerates the healing of ischemic intestinal injury by activating HIF-1α/PPARα pathway-mediated fatty acid oxidation
Cell Death Discovery (2024)
-
Intestinal IL-22RA1 signaling regulates intrinsic and systemic lipid and glucose metabolism to alleviate obesity-associated disorders
Nature Communications (2024)
-
SLE serum induces altered goblet cell differentiation and leakiness in human intestinal organoids
EMBO Molecular Medicine (2024)
-
FOXO transcription factors as mediators of stress adaptation
Nature Reviews Molecular Cell Biology (2024)
-
Emerging roles of mitochondrial functions and epigenetic changes in the modulation of stem cell fate
Cellular and Molecular Life Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.