Abstract
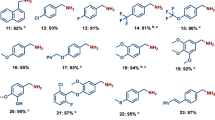
The development of catalyst-controlled stereoselective olefin metathesis processes1 has been a pivotal recent advance in chemistry. The incorporation of appropriate ligands within complexes based on molybdenum2, tungsten3 and ruthenium4 has led to reactivity and selectivity levels that were previously inaccessible. Here we show that molybdenum monoaryloxide chloride complexes furnish higher-energy (Z) isomers of trifluoromethyl-substituted alkenes through cross-metathesis reactions with the commercially available, inexpensive and typically inert Z-1,1,1,4,4,4-hexafluoro-2-butene. Furthermore, otherwise inefficient and non-stereoselective transformations with Z-1,2-dichloroethene and 1,2-dibromoethene can be effected with substantially improved efficiency and Z selectivity. The use of such molybdenum monoaryloxide chloride complexes enables the synthesis of representative biologically active molecules and trifluoromethyl analogues of medicinally relevant compounds. The origins of the activity and selectivity levels observed, which contradict previously proposed principles5, are elucidated with the aid of density functional theory calculations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoveyda, A. H., Khan, R. K. M., Torker, S. & Malcolmson, S. J. in Handbook of Metathesis (eds Grubbs, R. H. et al. ) 503–562 (Wiley–VCH, 2014)
Malcolmson, S. J., Meek, S. J., Sattely, E. S., Schrock, R. R. & Hoveyda, A. H. A new class of chiral catalysts for enantioselective alkene metathesis. Nature 456, 933–937 (2008)
Yu, M. et al. Synthesis of macrocyclic natural products by catalyst-controlled stereoselective ring-closing metathesis. Nature 479, 88–93 (2011)
Herbert, M. B. & Grubbs, R. H. Z-Selective cross metathesis with ruthenium catalysts: synthetic applications and mechanistic implications. Angew. Chem. Int. Ed . 54, 5018–5024 (2015)
Poater, A., Solans-Monfort, X., Copéret, C. & Eisenstein, O. Understanding d0-olefin metathesis catalysts: which metal, which ligands? J. Am. Chem. Soc. 129, 8207–8216 (2007)
Meek, S. J., O’Brien, R. V., Llaveria, J., Schrock, R. R. & Hoveyda, A. H. Catalytic Z-selective olefin cross-metathesis for natural product synthesis. Nature 471, 461–466 (2011)
Nguyen, T. T. et al. Kinetically E-selective olefin metathesis reactions. Science 352, 569–575 (2016)
Koh, M. J., Nguyen, T. T., Zhang, H., Schrock, R. R. & Hoveyda, A. H. Direct synthesis of Z-alkenyl halides through catalytic cross-methathesis. Nature 531, 459–465 (2016)
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015)
Innocenti, P. et al. Design of potent and selective hybrid inhibitors of the mitotic kinase Nek2: structure–activity relationship, structural biology, and cellular activity. J. Med. Chem. 55, 3228–3241 (2012)
Liu, X., Shimizu, M. & Hiyama, T. A facile stereocontrolled approach to CF3-substituted triarylethenes: synthesis of panomifene. Angew. Chem. Int. Ed . 43, 879–882 (2004)
Fujita, M., Hiyama, T. & Kondo, K. Practical and stereocontrolled synthesis of both (1R*,3S*)- and (1R*,3R*)-3-(2-chloro-3,3,3,-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylates. Tetrahedr. Lett . 27, 2139–2142 (1986)
Shimizu, M., Takeda, Y., Higashi, M. & Hiyama, T. Synthesis and photophysical properties of dimethoxybis(3,3,3-trifluoropropen-1-yl)benzenes: compact chromophores exhibiting violet fluorescence in the solid state. Chem. Asian J . 6, 2536–2544 (2011)
Hafner, A., Fischer, T. S. & Bräse, S. Synthesis of CF3-substituted olefins by Julia–Kocienski olefination using 2-[(2,2,2-trifluoroethyl)sulfonyl]benzo[d]thiazole as trifluoromethylation agent. Eur. J. Org. Chem. 2013, 7996–8003 (2013)
Choi, S. et al. Hydrotrifluoromethylation and iodotrifluoromethylation of alkenes and alkynes using an inorganic electride as a radical generator. Nat. Commun . 5, 4881 (2014)
Choi, W. J. et al. Mechanisms and applications of cyclometalated Pt(ii) complexes in photoredox catalytic trifluoromethylation. Chem. Sci . 6, 1454–1464 (2015)
Imhof, S., Randl, R. & Blechert, S. Ruthenium catalysed cross metathesis with fluorinated olefins. Chem. Commun . 2001, 1692–1693 (2001)
Ramachandran, P. V. & Mitsubishi, W. (Z)- or (E)-selective hydrogenation of potassium (3,3,3-trifluoroprop-1-yn-1-yl)trifluoroborate: route to either isomer of β-trifluoromethylstyrenes. Org. Lett. 17, 1252–1255 (2015)
Lin, Q.-Y., Xu, X.-H. & Qing, F.-L. Chemo-, regio-, and stereoselective trifluoromethylation of styrenes via visible light-driven single-electron transfer (SET) and triplet–triplet energy transfer (TTET) processes. J. Org. Chem. 79, 10434–10446 (2014)
Ichikawa, T., Kawasaki-Takasuka, T., Yamada, S. & Yamazaki, T. Construction of chiral trifluoromethylated materials by combination of stereochemically predictable SN2′ reaction and Ireland–Claisen rearrangement. J. Fluor. Chem. 152, 38–45 (2013)
Marinescu, S. C., Schrock, R. R., Li, B. & Hoveyda, A. H. Inversion of configuration at the metal in diastereomeric imido alkylidene monoaryloxide monopyrrolide complexes of molybdenum. J. Am. Chem. Soc. 131, 58–59 (2009)
Johansson Seechurn, C. C. C., Kitching, M. O., Colacot, T. J. & Sniekus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed . 51, 5062–5085 (2012)
Baasandorj, M., Ravishankara, A. R. & Burkholder, J. B. Atmospheric chemistry of (Z)-CF3CH=CHCF3: OH radical reaction rate coefficient and global warming potential. J. Phys. Chem. A 115, 10539–10549 (2011)
Mceachern, E. J., Vocadlo, D. J., Zhou, Y. & Selnick, H. G. Glycosidase inhibitors and uses thereof. Patent WO2014/032187 A1 (2014)
Kelly, M. et al. Amide derivatives as ion-channel ligands and pharmaceutical compositions and methods of using the same. Patent WO2006/093832 A2 (2006)
Zlatopolskiy, B. D., Kroll, H.-P., Melotto, E. & de Meijere, A. Convergent syntheses of N-Boc-protected (2S,4R)-4-(Z)-propenylproline and 5-chloro-1-(methoxymethoxy)pyrrol-2-carboxylic acid − two essential building blocks for the signal metabolite hormaomycin. Eur. J. Org. Chem . 4492–4502 (2004)
Hua, X.-Y., Chen, P., Hwang, J. & Yaksh, T. L. Antinociception induced by civamide, an orally active capsaicin analogue. Pain 71, 313–322 (1997)
English, A. R., Girard, D., Jasys, V. J., Martingano, R. J. & Kellogg, M. S. Orally effective acid prodrugs of the β-lactamase inhibitor sulbactam. J. Med. Chem. 33, 344–347 (1990)
Ramirez, M. A. & Borja, N. L. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy 28, 646–655 (2008)
Luo, X.-D. & Shen, C.-C. The chemistry, pharmacology, and clinical applications of qinghaosu (artemisinin) and its derivatives. Med. Res. Rev . 7, 29–52 (1987)
Acknowledgements
This research was supported by the United States National Institutes of Health, Institute of General Medical Sciences (GM-59426). M.J.K. acknowledges support through LaMattina and Bristol-Myers Squibb Graduate Fellowships. We thank P. Müller for helping to obtain various X-ray structures and X. Shen for advice and experimental assistance. We are grateful to XiMo, AG for gifts of paraffin tablets.
Author information
Authors and Affiliations
Contributions
M.J.K. and T.T.N. were involved in the discovery, design and development of the new Z-selective cross-metathesis strategies and their applications. J.K.L., J.H. and R.R.S. were involved in the synthesis and characterization of Mo MAC complexes. S.T. designed and performed the computational investigations, developed the models for the observed levels and patterns in reactivity and stereoselectivity. A.H.H. directed the investigation and wrote the manuscript with suggestions from M.J.K., T.T.N., J.K.L., S.T. and R.R.S.
Corresponding author
Ethics declarations
Competing interests
The catalysts and applications are licensed to a company (XiMo, AG) founded by A.H.H. and R.R.S.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Tables 1-4 and additional references (see Contents for details). (PDF 25285 kb)
Rights and permissions
About this article
Cite this article
Koh, M., Nguyen, T., Lam, J. et al. Molybdenum chloride catalysts for Z-selective olefin metathesis reactions. Nature 542, 80–85 (2017). https://doi.org/10.1038/nature21043
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21043
This article is cited by
-
Stereodefined alkenes with a fluoro-chloro terminus as a uniquely enabling compound class
Nature Chemistry (2022)
-
Air-stable 18-electron adducts of Schrock catalysts with tuned stability constants for spontaneous release of the active species
Communications Chemistry (2021)
-
Synthesis of E- and Z-trisubstituted alkenes by catalytic cross-metathesis
Nature (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.