Abstract
XRCC1 is a molecular scaffold protein that assembles multi-protein complexes involved in DNA single-strand break repair1,2. Here we show that biallelic mutations in the human XRCC1 gene are associated with ocular motor apraxia, axonal neuropathy, and progressive cerebellar ataxia. Cells from a patient with mutations in XRCC1 exhibited not only reduced rates of single-strand break repair but also elevated levels of protein ADP-ribosylation. This latter phenotype is recapitulated in a related syndrome caused by mutations in the XRCC1 partner protein PNKP3,4,5 and implicates hyperactivation of poly(ADP-ribose) polymerase/s as a cause of cerebellar ataxia. Indeed, remarkably, genetic deletion of Parp1 rescued normal cerebellar ADP-ribose levels and reduced the loss of cerebellar neurons and ataxia in Xrcc1-defective mice, identifying a molecular mechanism by which endogenous single-strand breaks trigger neuropathology. Collectively, these data establish the importance of XRCC1 protein complexes for normal neurological function and identify PARP1 as a therapeutic target in DNA strand break repair-defective disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Caldecott, K. W. Single-strand break repair and genetic disease. Nature Rev. Genet. 9, 619–631 (2008)
Caldecott, K. W. XRCC1 and DNA strand break repair. DNA Repair (Amst.) 2, 955–969 (2003)
Shen, J. et al. Mutations in PNKP cause microcephaly, seizures and defects in DNA repair. Nature Genet. 42, 245–249 (2010)
Bras, J. et al. Mutations in PNKP cause recessive ataxia with oculomotor apraxia type 4. Am. J. Hum. Genet. 96, 474–479 (2015)
Poulton, C. et al. Progressive cerebellar atrophy and polyneuropathy: expanding the spectrum of PNKP mutations. Neurogenetics 14, 43–51 (2013)
Caldecott, K. W., Tucker, J. D., Stanker, L. H. & Thompson, L. H. Characterization of the XRCC1-DNA ligase III complex in vitro and its absence from mutant hamster cells. Nucleic Acids Res. 23, 4836–4843 (1995)
Lee, Y. et al. The genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Nature Neurosci. 12, 973–980 (2009)
Tebbs, R. S. et al. Requirement for the Xrcc1 DNA base excision repair gene during early mouse development. Dev. Biol. 208, 513–529 (1999)
Tebbs, R. S., Thompson, L. H. & Cleaver, J. E. Rescue of Xrcc1 knockout mouse embryo lethality by transgene-complementation. DNA Repair (Amst.) 2, 1405–1417 (2003)
Bradley, M. O. & Kohn, K. W. X-ray induced DNA double strand break production and repair in mammalian cells as measured by neutral filter elution. Nucleic Acids Res. 7, 793–804 (1979)
Dillehay, L. E., Thompson, L. H., Minkler, J. L. & Carrano, A. V. The relationship between sister-chromatid exchange and perturbations in DNA replication in mutant EM9 and normal CHO cells. Mutat. Res. 109, 283–296 (1983)
Takashima, H. et al. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nature Genet. 32, 267–272 (2002)
El-Khamisy, S. F. et al. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 434, 108–113 (2005)
Moreira, M. C. et al. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nature Genet. 29, 189–193 (2001)
Eliasson, M. J. et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nature Med. 3, 1089–1095 (1997)
Chiarugi, A. Poly(ADP-ribose) polymerase: killer or conspirator? The ‘suicide hypothesis’ revisited. Trends Pharmacol. Sci. 23, 122–129 (2002)
Katyal, S. et al. Aberrant topoisomerase-1 DNA lesions are pathogenic in neurodegenerative genome instability syndromes. Nature Neurosci. 17, 813–821 (2014)
Marras, C. et al. Nomenclature of genetic movement disorders: recommendations of the International Parkinson and Movement Disorder Society task force. Mov. Disord. 31, 436–457 (2016)
Tetreault, M. et al. Whole-exome sequencing identifies novel ECHS1 mutations in Leigh syndrome. Hum. Genet. 134, 981–991 (2015)
Cappelli, E. et al. Involvement of XRCC1 and DNA ligase III gene products in DNA base excision repair. J. Biol. Chem. 272, 23970–23975 (1997)
Breslin, C. & Caldecott, K. W. DNA 3′-phosphatase activity is critical for rapid global rates of single-strand break repair following oxidative stress. Mol. Cell. Biol. 29, 4653–4662 (2009)
Fu, Y., Sander, J. D., Reyon, D., Cascio, V. M. & Joung, J. K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nature Biotechnol. 32, 279–284 (2014)
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013)
Pinkel, D., Thompson, L. H., Gray, J. W. & Vanderlaan, M. Measurement of sister chromatid exchanges at very low bromodeoxyuridine substitution levels using a monoclonal antibody in Chinese hamster ovary cells. Cancer Res. 45, 5795–5798 (1985)
Breslin, C. et al. Measurement of chromosomal DNA single-strand breaks and replication fork progression rates. Methods Enzymol. 409, 410–425 (2006)
Wang, Z. Q. et al. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 9, 509–520 (1995)
Acknowledgements
This work was funded by MRC Programme Grants (MR/J006750/1 and MR/P010121/1) to K.W.C., a ‘Science without Borders’ postdoctoral fellowship (CAPES Foundation, Ministry of Education, Brazil, BEX9769-13-7) to N.H., and funding to G.Y. from Genome Canada, the Canadian Institutes of Health Research, the Ontario Genomics Institute, Ontario Research Fund, Genome Quebec, the Children’s Hospital of Eastern Ontario Foundation, and the Hospital for Sick Children. K.S. was funded by the BBSRC grant BB/K019015/1 and P.J.M. acknowledges the National Institutes of Health (NS-37956, CA-21765), the CCSG (P30 CA21765), and the American Lebanese and Syrian Associated Charities of St. Jude Children’s Research Hospital for support. We thank the patient and her family for their contribution to this study. This work was selected for study by the Care4Rare Canada (Enhanced Care for Rare Genetic Diseases in Canada) Consortium Gene Discovery Steering Committee (for committee members, see below). We thank D. Dyment for his advice and discussion. We thank S. van der Velde-Visser and J. Schuurs-Hoeijmakers for Epstein-Barr virus transformation of the patient’s and sibling’s LCLs. We also thank S. El-Khamisy and A. Ridley for preliminary analyses and assistance with the mouse work.
Author information
Authors and Affiliations
Consortia
Contributions
H.H. generated gene-edited RPE-1 cell lines and designed and conducted the immunofluorescence, high-content imaging (Olympus ScanR), protein complementation, and double-strand break repair experiments. N.H. analysed XRCC1-patient cells by western blotting and RT-qPCR and designed and conducted sister chromatid exchange and comet assays. S.L.R., E.K., and L.J. designed and conducted mouse behaviour and histopathology experiments. S.R. and K.S. designed and conducted electrophysiology experiments. P.H. prepared recombinant XRCC1. Z.Z. generated CRISPR guide constructs. W.G. conducted preliminary CPT/ADP-ribose experiments. G.M.S.M. provided PNKP-patient fibroblasts. P.J.M. and Z.-Q.W. provided mouse models. G.Y. identified and oversaw genetic analysis of the patient, and J.W. and M.T. conducted exome analysis. K.W.C. conceived and managed the project, and wrote the manuscript with H.H. and N.H. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
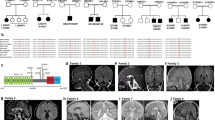
Extended Data Figure 1 Aberrant splicing of XRCC1 pre-mRNA in XRCC1-patient cells.
a, qPCR analysis of cDNA prepared from polyA-tailed RNA from WT, unaffected sibling, and patient LCLs. Cells were mock-treated or treated with cycloheximide (CHX) to inhibit nonsense-mediated decay. Cartoons show the position of primers used to quantify total XRCC1 mRNA (left), mRNA with correctly spliced exon11/12 junction (middle), or intron 11 retention (right). Fold change was calculated from ΔΔCt values relative to actin and untreated WT from three independent experiments (mean ± s.e.m.). b, Summary of splicing defects observed in sequenced XRCC1 transcripts from sibling and patient cells treated with CHX. Whole XRCC1 cDNA was amplified from oligodT-primed reverse-transcribed RNA and individual transcripts cloned and sequenced. The allelic origin of individual patient transcripts was assigned using a R399Q SNP in the Q465X allele.
Extended Data Figure 2 XRCC1 and Lig3α protein levels in XRCC1−/− RPE-1 cells and XRCC1-patient cells.
Quantification of (a) XRCC1 and (b) Lig3α protein levels in the indicated cell lines by western blotting. Data are the mean signal intensity from three independent experiments (± s.e.m.) normalized to tubulin and to the respective WT sample for each cell type. The anti-XRCC1 signal detected in XRCC1−/− RPE-1 cells reflects non-specific background.
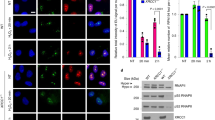
Extended Data Figure 3 Reduced XRCC1 recruitment into damaged chromatin in XRCC1-patient cells.
a, XRCC1 recruitment into chromatin was compared in the indicated cell lines by ScanR high-content imaging before and 10 min after treatment with 1 mM H2O2. Cells were pre-extracted with detergent before fixation and immunostaining. Representative images of the ScanR (Olympus) data used for the quantification in Fig. 2d are shown. b, XRCC1 recruitment into chromatin using a high-resolution Zeiss microscope was compared in the indicated cell lines after treatment 45 min with 30 μM CPT. Cells were pre-extracted with detergent before fixation and immunostaining as above. Scale bars, 10 μm.
Extended Data Figure 4 Representative images from ScanR high-content imaging of γ-H2AX foci (green) in the nuclei (blue) of the indicated cells at the indicated times after ionizing radiation (2 Gy).
Quantification is shown in Fig. 3b.
Extended Data Figure 5 Elevated ADP-ribosylation in XRCC1−/− RPE-1 cells, XRCC1-patient fibroblasts, and PNKP-patient fibroblasts.
a, ADP-ribosylated proteins were detected in cell extracts from WT RPE-1 cells, XRCC1−/− RPE-1 cells, WT 1BR fibroblasts, XRCC1-patient fibroblasts (‘X1 patient’), and PNKP-patient fibroblasts treated as in Fig. 4a by western blotting and Anti-pan-ADP-ribose binding reagent. b, Levels of ADP-ribosylation in 1BR WT, XRCC1-patient, and PNKP-patient fibroblasts measured before and after CPT treatment by indirect immunofluorescence as indicated in Fig. 4b. Representative ScanR images are shown.
Extended Data Figure 6 Elevated ADP-ribose levels in CPT-treated XRCC1−/− RPE-1 cells are PARP1 dependent.
a, Representative ScanR images of WT, XRCC1−/−, PARP1−/−, and XRCC1−/−/PARP1−/− RPE-1 cells before and after 30 μM CPT treatment stained by indirect immunofluorescence for XRCC1, PARP1, DAPI, or ADP-ribose. b, Quantification of ADP-ribose intensity in the nucleus from >3,500 cells per sample from a (data from single experiment). c, Western blot showing XRCC1 and PARP1 protein levels in the indicated RPE-1 cell lines.
Extended Data Figure 7 Elevated ADP-ribose levels in CPT-treated XRCC1-patient and PNKP-patient cells are prevented by PARP inhibitors.
Levels of ADP-ribose in WT 1BR fibroblasts, XRCC1-patient fibroblasts and PNKP-patient fibroblasts were measured before and after 45 min 30 μM CPT treatment, and after 45 min 30 μM CPT treatment in the presence of 10 μM of the indicated PARP inhibitor (also including 1 h pre-treatment). Cells were pre-extracted with detergent before fixation and immunostaining with Anti-pan-ADP-ribose binding reagent. Representative ScanR images are shown in a and quantification from more than 1,500 cells per sample, from a single experiment, are shown in b.
Extended Data Figure 8 Levels of ADP-ribosylation in XRCC1-patient cells transfected with XRCC1 protein.
Levels of ADP-ribosylation were quantified before and after CPT treatment by high content imaging in WT 1BR fibroblasts, XRCC1-patient fibroblasts, and XRCC1-patient fibroblasts transfected by electroporation in the presence of 1 μg or 2 μg of control BSA or purified recombinant human XRCC1-His protein. Representative ScanR images are presented. Quantification of ADP-ribosylation is shown in Fig. 4d.
Extended Data Figure 9 Reduced cerebellar Purkinje cell firing frequency in Xrcc1Nes-Cre (KO) mice.
a, Representative traces from cell-attached recordings. b, Summary of data. Bars and error bars indicate mean ± s.e.m. firing frequencies for Xrcc1 WT (13.35 ± 1.73, n = 14 cells) versus Xrcc1Nes-Cre mice (8.96 ± 0.82, n = 18 cells) (*P < 0.05, t-test). Circles are mean firing frequencies for each cell.
Extended Data Figure 10 Chromosomal SSBR rates in the indicated RPE-1 cell lines after treatment with 50 μM H2O2 in the presence/absence of 10 μM of the PARP inhibitor (PARPi) Veliparib, as measured by alkaline comet assay.
Data are the mean tail moment of 100 cells per sample per experiment and are the average (± s.e.m.) of three independent experiments. Two-way analysis of variance between relevant genotypes is presented (NS, not significant, **P < 0.01, ***P < 0.001).
Supplementary information
Supplementary Figures
This file contains the uncropped blots for Figures 2b and 4b. (PDF 2559 kb)
Supplementary Information
This file contains Supplementary Text and Data. (PDF 125 kb)
Rights and permissions
About this article
Cite this article
Hoch, N., Hanzlikova, H., Rulten, S. et al. XRCC1 mutation is associated with PARP1 hyperactivation and cerebellar ataxia. Nature 541, 87–91 (2017). https://doi.org/10.1038/nature20790
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20790
This article is cited by
-
Increased cysteinyl-tRNA synthetase drives neuroinflammation in Alzheimer’s disease
Translational Neurodegeneration (2024)
-
Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases
Translational Neurodegeneration (2023)
-
PARP1 and XRCC1 exhibit a reciprocal relationship in genotoxic stress response
Cell Biology and Toxicology (2023)
-
Deficient DNA base-excision repair in the forebrain leads to a sex-specific anxiety-like phenotype in mice
BMC Biology (2022)
-
A mechanism for oxidative damage repair at gene regulatory elements
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.