Abstract
HAND2 is an ancestral regulator of heart development and one of four transcription factors that control the reprogramming of fibroblasts into cardiomyocytes1,2,3,4. Deletion of Hand2 in mice results in right ventricle hypoplasia and embryonic lethality1,5. Hand2 expression is tightly regulated by upstream enhancers6,7 that reside within a super-enhancer delineated by histone H3 acetyl Lys27 (H3K27ac) modifications8. Here we show that transcription of a Hand2-associated long non-coding RNA, which we named upperhand (Uph), is required to maintain the super-enhancer signature and elongation of RNA polymerase II through the Hand2 enhancer locus. Blockade of Uph transcription, but not knockdown of the mature transcript, abolished Hand2 expression, causing right ventricular hypoplasia and embryonic lethality in mice. Given the substantial number of uncharacterized promoter-associated long non-coding RNAs encoded by the mammalian genome9, the Uph–Hand2 regulatory partnership offers a mechanism by which divergent non-coding transcription can establish a permissive chromatin environment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Srivastava, D. et al. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat. Genet. 16, 154–160 (1997)
Han, Z., Yi, P., Li, X. & Olson, E. N. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development 133, 1175–1182 (2006)
Song, K. et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604 (2012)
Conway, S. J., Firulli, B. & Firulli, A. B. A bHLH code for cardiac morphogenesis. Pediatr. Cardiol. 31, 318–324 (2010)
Yamagishi, H., Olson, E. N. & Srivastava, D. The basic helix-loop-helix transcription factor, dHAND, is required for vascular development. J. Clin. Invest. 105, 261–270 (2000)
Charité, J. et al. Role of Dlx6 in regulation of an endothelin-1-dependent, dHAND branchial arch enhancer. Genes Dev. 15, 3039–3049 (2001)
McFadden, D. G. et al. A GATA-dependent right ventricular enhancer controls dHAND transcription in the developing heart. Development 127, 5331–5341 (2000)
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013)
Lepoivre, C. et al. Divergent transcription is associated with promoters of transcriptional regulators. BMC Genomics 14, 914 (2013)
Voth, H. et al. Co-regulated expression of HAND2 and DEIN by a bidirectional promoter with asymmetrical activity in neuroblastoma. BMC Mol. Biol. 10, 28 (2009)
Chodroff, R. A. et al. Long noncoding RNA genes: conservation of sequence and brain expression among diverse amniotes. Genome Biol. 11, R72 (2010)
Kambara, H. et al. Regulation of interferon-stimulated gene BST2 by a lncRNA transcribed from a shared bidirectional promoter. Front. Immunol. 5, 676 (2015)
Uesaka, M. et al. Bidirectional promoters are the major source of gene activation-associated non-coding RNAs in mammals. BMC Genomics 15, 35 (2014)
Anderson, D. M. et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 160, 595–606 (2015)
Nelson, B. R. et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351, 271–275 (2016)
Grote, P. et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell 24, 206–214 (2013)
Wang, K. C. et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 (2011)
Yang, Y. W. et al. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. eLife 3, e02046 (2014)
Stein, C. A. et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 38, e3 (2010)
Kaikkonen, M. U. et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol. Cell 51, 310–325 (2013)
Kim, T. K. et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 465, 182–187 (2010)
Schmitt, S., Prestel, M. & Paro, R. Intergenic transcription through a polycomb group response element counteracts silencing. Genes Dev. 19, 697–708 (2005)
Herzog, V. A. et al. A strand-specific switch in noncoding transcription switches the function of a Polycomb/Trithorax response element. Nat. Genet. 46, 973–981 (2014)
Ho, Y., Elefant, F., Liebhaber, S. A. & Cooke, N. E. Locus control region transcription plays an active role in long-range gene activation. Mol. Cell 23, 365–375 (2006)
He, A. et al. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat. Commun. 5, 4907 (2014)
Jonkers, I. & Lis, J. T. Getting up to speed with transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 16, 167–177 (2015)
Yanagisawa, H., Clouthier, D. E., Richardson, J. A., Charité, J. & Olson, E. N. Targeted deletion of a branchial arch-specific enhancer reveals a role of dHAND in craniofacial development. Development 130, 1069–1078 (2003)
Barron, F. et al. Downregulation of Dlx5 and Dlx6 expression by Hand2 is essential for initiation of tongue morphogenesis. Development 138, 2249–2259 (2011)
Charité, J., McFadden, D. G. & Olson, E. N. The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development 127, 2461–2470 (2000)
Lucas, M. E., Müller, F., Rüdiger, R., Henion, P. D. & Rohrer, H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development 133, 4015–4024 (2006)
Anderson, D. M. et al. Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev. Dyn. 235, 792–801 (2006)
Shelton, J. M., Lee, M. H., Richardson, J. A. & Patel, S. B. Microsomal triglyceride transfer protein expression during mouse development. J. Lipid Res. 41, 532–537 (2000)
Gagnon, K. T., Li, L., Janowski, B. A. & Corey, D. R. Analysis of nuclear RNA interference in human cells by subcellular fractionation and Argonaute loading. Nat. Protocols 9, 2045–2060 (2014)
Conrad, N. K. Chapter 15. Co-immunoprecipitation techniques for assessing RNA-protein interactions in vivo. Methods Enzymol. 449, 317–342 (2008)
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012)
Acknowledgements
We thank D. Tennison for technical assistance and J. Cabrera for graphics. We thank the Histopathology Core Facility at University of Texas Southwestern for histology. This work was supported by grants from the National Institutes of Health (HL-077439, AR-067294, HL-130253, DK-099653 and U01-HL-100401), Fondation Leducq Networks of Excellence, Cancer Prevention and Research Institute of Texas and the Robert A. Welch Foundation (grant 1-0025 to E.N.O.), a pre-doctoral fellowship from the American Heart Association (14PRE19830031) to K.M.A. and a Muscular Dystrophy Association Development Grant (MDA377340) to D.M.A.
Author information
Authors and Affiliations
Contributions
K.M.A., D.M.A. and E.N.O. designed experiments and analysed data. J.R.M. generated mutant mice from constructs designed and created by K.M.A. and D.M.A. K.M.A. performed qPCR, phenotypic analysis, dissections, ChIP, RNA immunoprecipitation, cardiomyocyte fractionation, Southern blot, and India ink injections. D.M.A performed northern blot, RACE, in vitro transcription/translation, and GapmeR transfections. J.M.S. and K.M.A. performed in situ hybridization and generated images. K.M.A., D.M.A, E.N.O. and R.B.D. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks H. Chang and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
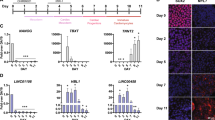
Extended Data Figure 1 Sequence alignment of several mammalian Uph transcripts.
a, Sequence alignment of several mammalian Uph transcripts performed using ClustalW. See Methods for source data. b, Diagram of the Hand2 locus in mammals showing the genomic organization and orientation of Uph. The Hand2 branchial arch enhancer (green) and cardiac enhancer (yellow) are shown. c, Diagram of the Uph transcripts expressed in the mouse heart, determined using 5′ and 3′ RACE using primers specific to exon 4 of Uph. AP1, marathon adaptor primer; 3′GSP, 3′ Uph-specific primer from exon 4; 5′GSP, 5′ Uph-specific primer from exon 4. d, Whole mount in situ hybridization of E10.5 mouse embryos. Expression was detected in heart, branchial arches (arrowhead), and limb bud. Scale bars, 1 mm. e, Northern blot analysis of total RNA from adult mouse tissues using a probe specific to the major Uph transcript. For gel source data, see Supplementary Fig. 1.
Extended Data Figure 2 Uph is a cytoplasmic lncRNA.
a, Subcellular fractionation of 18S, Uph and Malat1 lncRNA in mouse neonatal cardiomyocytes (n = 3 biological replicates from 1 of 5 independent experiments; mean ± s.e.m.). b, In vitro transcription and translation of a plasmid encoding the major Uph RNA. A plasmid encoding the myoregulin (MLN) micropeptide was used as a positive control, and myoregulin with a frameshift mutation (MLN RNA-FS) was used as a negative control. In contrast to myoregulin, Uph and the negative control (MLN RNA-FS) did not produce any detectable proteins, indicating that Uph is a bona fide lncRNA. For gel source data, see Supplementary Fig. 1.
Extended Data Figure 3 Targeting strategy for insertion of transcriptional termination or heterologous sequence into exon 2 of Uph.
a, Uph KO targeting strategy. Transcription activator-like effector nucleases (TALENs) were used to insert a triple polyadenylation (tpA) termination sequence into exon 2 (E2) of Uph. b, Using the same TALEN pair as in a, we introduced the coding sequence of tdTO, lacking a polyadenylation sequence, into exon 2 of the Uph locus. Exon 2, which includes the tdTO coding sequence, was spliced out of the mature Uph transcript, preventing expression of tdTO in these mice. c, Southern blot analysis of wild-type, heterozygous Uph+/− (Het) and Uph KO genomic DNA. BamHI-digested DNA hybridized with a 5′-specific probe and NdeI-digested DNA hybridized with a 3′-specific probe. For gel source data, see Supplementary Fig. 1. d, Southern blot analysis of wild-type, UphtdTO/+ heterozygous and UphtdTO/tdTO knock-in (KI) genomic DNA verified the correct targeting of the tdTO sequence into the Uph locus. DNA was digested with BamHI and hybridized with a 5′-specific probe or digested with NdeI and hybridized with a 3′-specific probe. For gel source data, see Supplementary Fig. 1. e, f, Expression of Uph (e) and Hand2 (f) in wild-type and UphtdTO/tdTO homozygous mice at E10.0 was not changed by the insertion of the tdTO sequence into exon 2 of the Uph locus (n = 3, representative of 3 independent experiments; mean ± s.e.m.). g, qPCR shows Uph transcripts decreased by ~97% in E10.5 hearts (n = 3 mice of each genotype from 1 of 3 independent experiments; mean ± s.e.m.).
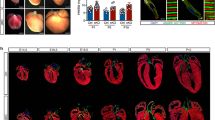
Extended Data Figure 4 Aortic arch arteries are normal in Uph KO embryos.
a, qPCR quantification of gene expression at E10.0 showed robust downregulation of Uph and Hand2 expression in Uph KO hearts, with normal expression of other cardiac transcription factors (n = 3 mice of each genotype from 1 of 3 independent experiments; mean ± s.e.m.). b, India ink was injected into either the left ventricle of wild-type embryos or the single ventricle of Uph KO embryos at E10.5, to visualize the aortic arch arteries and circulation, which appeared normal in Uph KO embryos. aa, aortic arch arteries; as, aortic sac; da, dorsal aorta. Scale bars, 1 mm.
Extended Data Figure 5 Uph+/− Hand2+/− compound heterozygotes recapitulate Uph KO phenotype.
a, Uph+/− Hand2+/− double heterozygote embryos developed a single ventricle, pericardial effusion, and were severely growth restricted by E11.5. Scale bars, 1 mm. b, Uph expression is normal in double heterozygotes, whereas Hand2 was reduced by ~90%, relative to wild-type embryos (n = 5 mice for wild type, Hand2 het and Uph het, n = 3 for double het, n = 2 for Hand2 KO, from 1 of 2 independent experiments; mean ± s.e.m.). c, Immunoprecipitation of RNA using either IgG or WDR5 in HL-1 cells followed by qPCR for Uph revealed no binding to WDR5. The WDR5-interacting lncRNA HOTTIP was used as a positive control (n = 3 biological replicates from 1 of 3 independent experiments; mean ± s.e.m.).
Extended Data Figure 6 Knockdown of mature Uph transcripts in HL-1 or Neuro2a cells does not affect Hand2 expression.
a, Expression of Uph (red) and Hand2 (blue) in various tissues and cell lines. Uph and Hand2 are robustly expressed in the heart, and the HL-1 and Neuro2a cell lines. Uph and Hand2 transcripts are not expressed in the liver, 10T1/2 fibroblasts or skeletal muscle C2C12 myotubes (n = 3 technical replicates; mean ± s.e.m.). b, c, Uph transcripts were reduced by ~90% in HL-1 (b) and Neuro2a (c) cells when transfected with two independent GapmeR antisense oligonucleotide probes (ASO A and B) against Uph. Expression of Hand2 was not changed (n = 2 for ASO A and B, n = 3 for control, from 1 of 2 independent experiments; mean ± s.e.m.). d, Uph transcripts were similarly downregulated across each exon–exon junction, measured using qPCR (n = 2 for ASO A and B, n = 3 for control, from 1 of 2 independent experiments; mean ± s.e.m.). e, Fractionation of HL-1 cells transfected with control or Uph-specific ASOs. The nuclear fraction of antisense-oligonucleotide-treated HL-1 cells showed a similar downregulation of Uph transcripts (n = 3 biological replicates from 1 of 3 independent experiments; mean ± s.e.m.). f, Overexpression of enhanced green fluorescent protein (eGFP; blue), the major Uph RNA (red) or Hand2 RNA (green) in HL-1 cells revealed that the mature Uph transcript did not alter Hand2 expression relative to wild type, and that Hand2 does not influence the expression of Uph in these cells (n = 2 biological replicates from 1 of 2 independent experiments; mean ± s.e.m.).
Extended Data Figure 7 ChIP and qPCR analyses of active cardiac enhancers regulating Nkx2-5 expression.
a, Diagram of the Nkx2-5 locus with numbers (1 and 2) indicating the region analysed by qPCR following ChIP. Shown in red are the ENCODE/LICR H3K4me1 active enhancer marks in the heart at E14.5. See Methods for source data. b, ChIP coupled with qPCR analysis of H3K4me1 marks, normalized to total histone H3, showed that the H3K4me1 marks bound to the Nkx2-5 promoter region are unchanged between wild-type, Uph KO and UphtdTO/tdTO homozygous hearts at E10.0. c, ChIP coupled with qPCR analysis of H3K27ac marks, normalized to total histone H3, showed that the H3K27ac marks bound to the Nkx2-5 promoter region are unchanged between wild-type, Uph KO and UphtdTO/tdTO homozygous hearts at E10.0. d, GATA4 binding to a GATA4 site in the Nkx2-5 promoter is unchanged between wild-type and Uph KO hearts at E10.0. e, Diagram of the Uph–Hand2 locus, with black bars indicating the regions analysed by qPCR following ChIP. Shown in red is the ENCODE/LICR H3K27me3 track for mouse heart. See Methods for source data. The branchial arch enhancer (green) and cardiac enhancer (yellow) are shown. f, ChIP coupled with qPCR analysis of the polycomb repressive marker H3K27me3, normalized to total histone H3, showed no differences between genotypes at each locus (1–3) indicated in the diagram in e. g, qPCR revealed no difference in the levels of Ser2-phosphorylated RNAPII between genotypes at the Nkx2-5 gene body. Each point is one of 3 technical replicates of 5 pooled hearts for each genotype in each ChIP experiment, from 1 of 2 independent experiments; mean ± s.e.m.
Supplementary information
Supplementary Information
This file contains the Source data for Extended Data Figures 1e, 2d, 3c, 3d. (PDF 1082 kb)
Rights and permissions
About this article
Cite this article
Anderson, K., Anderson, D., McAnally, J. et al. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539, 433–436 (2016). https://doi.org/10.1038/nature20128
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20128
This article is cited by
-
Copy number variation-associated lncRNAs may contribute to the etiologies of congenital heart disease
Communications Biology (2023)
-
Epigenetic regulation in hematopoiesis and its implications in the targeted therapy of hematologic malignancies
Signal Transduction and Targeted Therapy (2023)
-
LNCGM1082-mediated NLRC4 activation drives resistance to bacterial infection
Cellular & Molecular Immunology (2023)
-
Long noncoding RNAs as versatile molecular regulators of cellular stress response and homeostasis
Human Genetics (2023)
-
A hidden translatome in tumors—the coding lncRNAs
Science China Life Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.