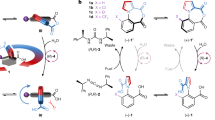
Abstract
Molecular machines are among the most complex of all functional molecules and lie at the heart of nearly every biological process1. A number of synthetic small-molecule machines have been developed2, including molecular muscles3,4, synthesizers5,6, pumps7,8,9, walkers10, transporters11 and light-driven12,13,14,15,16 and electrically17,18 driven rotary motors. However, although biological molecular motors are powered by chemical gradients or the hydrolysis of adenosine triphosphate (ATP)1, so far there are no synthetic small-molecule motors that can operate autonomously using chemical energy (that is, the components move with net directionality as long as a chemical fuel is present)19. Here we describe a system in which a small molecular ring (macrocycle) is continuously transported directionally around a cyclic molecular track when powered by irreversible reactions of a chemical fuel, 9-fluorenylmethoxycarbonyl chloride. Key to the design is that the rate of reaction of this fuel with reactive sites on the cyclic track is faster when the macrocycle is far from the reactive site than when it is near to it. We find that a bulky pyridine-based catalyst promotes carbonate-forming reactions that ratchet the displacement of the macrocycle away from the reactive sites on the track. Under reaction conditions where both attachment and cleavage of the 9-fluorenylmethoxycarbonyl groups occur through different processes, and the cleavage reaction occurs at a rate independent of macrocycle location, net directional rotation of the molecular motor continues for as long as unreacted fuel remains. We anticipate that autonomous chemically fuelled molecular motors will find application as engines in molecular nanotechnology2,19,20.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schliwa, M. & Woehlke, G. Molecular motors. Nature 422, 759–765 (2003)
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev. 115, 10081–10206 (2015)
Jiménez, M. C., Dietrich-Buchecker, C. & Sauvage, J.-P. Towards synthetic molecular muscles: contraction and stretching of a linear rotaxane dimer. Angew. Chem. Int. Ed. 39, 3284–3287 (2000)
Bruns, C. J. & Stoddart, J. F. Rotaxane-based molecular muscles. Acc. Chem. Res. 47, 2186–2199 (2014)
Thordarson, P., Bijsterveld, E. J. A., Rowan, A. E. & Nolte, R. J. M. Epoxidation of polybutadiene by a topologically linked catalyst. Nature 424, 915–918 (2003)
Lewandowski, B. et al. Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339, 189–193 (2013)
Serreli, V., Lee, C.-F., Kay, E. R. & Leigh, D. A. A molecular information ratchet. Nature 445, 523–527 (2007)
Ragazzon, G., Baroncini, M., Silvi, S., Venturi, M. & Credi, A. Light-powered autonomous and directional molecular motion of a dissipative self-assembling system. Nature Nanotechnol. 10, 70–75 (2014)
Cheng, C. et al. An artificial molecular pump. Nature Nanotechnol. 10, 547–553 (2015)
von Delius, M., Geertsema, E. M. & Leigh, D. A. A synthetic small molecule that can walk down a track. Nature Chem. 2, 96–101 (2010)
Kassem, S., Lee, A. T. L., Leigh, D. A., Markevicius, A. & Solà, J. Pick-up, transport and release of a molecular cargo using a small-molecule robotic arm. Nature Chem. 8, 138–143 (2016)
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999)
Eelkema, R. et al. Molecular machines: nanomotor rotates microscale objects. Nature 440, 163 (2006)
Greb, L. & Lehn, J.-M. Light-driven molecular motors: imines as four-step or two-step unidirectional rotors. J. Am. Chem. Soc. 136, 13114–13117 (2014)
Li, Q. et al. Macroscopic contraction of a gel induced by the integrated motion of light-driven molecular motors. Nature Nanotechnol. 10, 161–165 (2015)
Greb, L., Eichhöfer, A. & Lehn, J.-M. Synthetic molecular motors: thermal N inversion and directional photoinduced C=N bond rotation of camphorquinone imines. Angew. Chem. Int. Ed. 54, 14345–14348 (2015)
Tierney, H. L. et al. Experimental demonstration of a single-molecule electric motor. Nature Nanotechnol. 6, 625–629 (2011)
Perera, U. G. E. et al. Controlled clockwise and anticlockwise rotational switching of a molecular motor. Nature Nanotechnol. 8, 46–51 (2012)
Kay, E. R. & Leigh, D. A. Rise of the molecular machines. Angew. Chem. Int. Ed. 54, 10080–10088 (2015)
Astumian, R. D. Microscopic reversibility as the organizing principle of molecular machines. Nature Nanotechnol. 7, 684–688 (2012)
Feynman, R. P., Leighton, R. B. & Sands, M. The Feynman Lectures on Physics Vol. 1, Ch. 46 (Addison-Wesley, 1963)
Kelly, T. R., Tellitu, I. & Sestelo, J. P. In search of molecular ratchets. Angew. Chem. Int. Edn Engl. 36, 1866–1868 (1997)
Kelly, T. R., De Silva, H. & Silva, R. A. Unidirectional rotary motion in a molecular system. Nature 401, 150–152 (1999)
Kelly, T. R. et al. Progress toward a rationally designed, chemically powered rotary molecular motor. J. Am. Chem. Soc. 129, 376–386 (2007)
Leigh, D. A., Wong, J. K. Y., Dehez, F. & Zerbetto, F. Unidirectional rotation in a mechanically interlocked molecular rotor. Nature 424, 174–179 (2003)
Hernández, J. V., Kay, E. R. & Leigh, D. A. A reversible synthetic rotary molecular motor. Science 306, 1532–1537 (2004)
Fletcher, S. P., Dumur, F., Pollard, M. M. & Feringa, B. L. A reversible, unidirectional molecular rotary motor driven by chemical energy. Science 310, 80–82 (2005)
Haberhauer, G. A molecular four-stroke motor. Angew. Chem. Int. Ed. 50, 6415–6418 (2011)
Lu, C.-H., Cecconello, A., Elbaz, J., Credi, A. & Willner, I. A three-station DNA catenane rotary motor with controlled directionality. Nano Lett. 13, 2303–2308 (2013)
Davis, A. P. Tilting at windmills? The second law survives. Angew. Chem. Int. Ed. 37, 909–910 (1998)
Alvarez-Pérez, M., Goldup, S. M., Leigh, D. A. & Slawin, A. M. Z. A chemically-driven molecular information ratchet. J. Am. Chem. Soc. 130, 1836–1838 (2008)
Carlone, A., Goldup, S. M., Lebrasseur, N., Leigh, D. A. & Wilson, A. A three-compartment chemically-driven molecular information ratchet. J. Am. Chem. Soc. 134, 8321–8323 (2012)
Cheng, C., McGonigal, P. R., Stoddart, J. F. & Astumian, R. D. Design and synthesis of nonequilibrium systems. ACS Nano 9, 8672–8688 (2015)
Acknowledgements
We thank D. R. Astumian for the analysis of the catenane motor reaction kinetics, the European Research Council (ERC) for funding and the EPSRC National Mass Spectrometry Service Centre (Swansea, UK) for high-resolution mass spectrometry.
Author information
Authors and Affiliations
Contributions
M.R.W., A.C., J.S., S.M.G. and N.L. carried out the experimental work. M.R.W. and J.S. designed and performed the operation experiments. D.A.L. directed the research. All the authors contributed to the analysis of the results and the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Figures 1-77 and additional references (see Contents for more details). (PDF 10992 kb)
Rights and permissions
About this article
Cite this article
Wilson, M., Solà, J., Carlone, A. et al. An autonomous chemically fuelled small-molecule motor. Nature 534, 235–240 (2016). https://doi.org/10.1038/nature18013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18013
This article is cited by
-
A rhythmically pulsing leaf-spring DNA-origami nanoengine that drives a passive follower
Nature Nanotechnology (2024)
-
Catenane organocatalyst breaks the linear scaling limitation through conformational selection
Communications Chemistry (2024)
-
A catalytically active oscillator made from small organic molecules
Nature (2023)
-
Ratcheting synthesis
Nature Reviews Chemistry (2023)
-
An electric molecular motor
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.