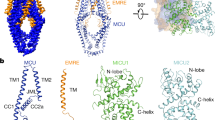
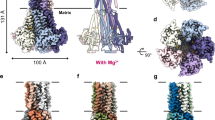
Abstract
Mitochondria from many eukaryotic clades take up large amounts of calcium (Ca2+) via an inner membrane transporter called the uniporter. Transport by the uniporter is membrane potential dependent and sensitive to ruthenium red or its derivative Ru360 (ref. 1). Electrophysiological studies have shown that the uniporter is an ion channel with remarkably high conductance and selectivity2. Ca2+ entry into mitochondria is also known to activate the tricarboxylic acid cycle and seems to be crucial for matching the production of ATP in mitochondria with its cytosolic demand3. Mitochondrial calcium uniporter (MCU) is the pore-forming and Ca2+-conducting subunit of the uniporter holocomplex, but its primary sequence does not resemble any calcium channel studied to date. Here we report the structure of the pore domain of MCU from Caenorhabditis elegans, determined using nuclear magnetic resonance (NMR) and electron microscopy (EM). MCU is a homo-oligomer in which the second transmembrane helix forms a hydrophilic pore across the membrane. The channel assembly represents a new solution of ion channel architecture, and is stabilized by a coiled-coil motif protruding into the mitochondrial matrix. The critical DXXE motif forms the pore entrance, which features two carboxylate rings; based on the ring dimensions and functional mutagenesis, these rings appear to form the selectivity filter. To our knowledge, this is one of the largest membrane protein structures characterized by NMR, and provides a structural blueprint for understanding the function of this channel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Biological Magnetic Resonance Data Bank
Protein Data Bank
Data deposits
The atomic structure coordinate and structural constraints are deposited in the Protein Data Bank (PDB) under the accession number 5ID3. The chemical shift values are deposited in the Biological Magnetic Resonance Data Bank (BMRB) under the accession number 30021.
References
Gunter, T. E. & Pfeiffer, D. R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258, C755–C786 (1990)
Kirichok, Y., Krapivinsky, G. & Clapham, D. E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 (2004)
Denton, R. M. & McCormack, J. G. The role of calcium in the regulation of mitochondrial metabolism. Biochem. Soc. Trans. 8, 266–268 (1980)
Baughman, J. M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011)
Perocchi, F. et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467, 291–296 (2010)
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011)
Sancak, Y. et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342, 1379–1382 (2013)
Raffaello, A. et al. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 32, 2362–2376 (2013)
Kamer, K. J. & Mootha, V. K. The molecular era of the mitochondrial calcium uniporter. Nature Rev. Mol. Cell Biol. 16, 545–553 (2015)
Chaudhuri, D., Sancak, Y., Mootha, V. K. & Clapham, D. E. MCU encodes the pore conducting mitochondrial calcium currents. eLife 2, e00704 (2013)
Kovács-Bogdán, E. et al. Reconstitution of the mitochondrial calcium uniporter in yeast. Proc. Natl Acad. Sci. USA 111, 8985–8990 (2014)
Lee, Y. et al. Structure and function of the N-terminal domain of the human mitochondrial calcium uniporter. EMBO Rep. (2015)
Van Horn, W. D. et al. Solution nuclear magnetic resonance structure of membrane-integral diacylglycerol kinase. Science 324, 1726–1729 (2009)
Schnell, J. R. & Chou, J. J. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 451, 591–595 (2008)
OuYang, B. et al. Unusual architecture of the p7 channel from hepatitis C virus. Nature 498, 521–525 (2013)
Cheng, Y. et al. Single particle reconstructions of the transferrin-transferrin receptor complex obtained with different specimen preparation techniques. J. Mol. Biol. 355, 1048–1065 (2006)
Guskov, A. et al. Structural insights into the mechanisms of Mg2+ uptake, transport, and gating by CorA. Proc. Natl Acad. Sci. USA 109, 18459–18464 (2012)
Pfoh, R. et al. Structural asymmetry in the magnesium channel CorA points to sequential allosteric regulation. Proc. Natl Acad. Sci. USA 109, 18809–18814 (2012)
Eshaghi, S. et al. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science 313, 354–357 (2006)
Lunin, V. V. et al. Crystal structure of the CorA Mg2+ transporter. Nature 440, 833–837 (2006)
Hou, X., Pedi, L., Diver, M. M. & Long, S. B. Crystal structure of the calcium release-activated calcium channel Orai. Science 338, 1308–1313 (2012)
Bick, A. G., Calvo, S. E. & Mootha, V. K. Evolutionary diversity of the mitochondrial calcium uniporter. Science 336, 886 (2012)
Hassaine, G. et al. X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 512, 276–281 (2014)
Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J. Mol. Biol. 346, 967–989 (2005)
Lupas, A., Van Dyke, M. & Stock, J. Predicting coiled coils from protein sequences. Science 252, 1162–1164 (1991)
Sonnhammer, E. L., von Heijne, G. & Krogh, A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6, 175–182 (1998)
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360 (1996)
Slotboom, D. J., Duurkens, R. H., Olieman, K. & Erkens, G. B. Static light scattering to characterize membrane proteins in detergent solution. Methods 46, 73–82 (2008)
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007)
Ludtke, S. J., Baldwin, P. R. & Chiu, W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128, 82–97 (1999)
Guo, X. et al. Structural insight into autoinhibition and histone H3-induced activation of DNMT3A. Nature 517, 640–644 (2015)
Cong, Y., Kovacs, J. A. & Wriggers, W. 2D fast rotational matching for image processing of biophysical data. J. Struct. Biol. 144, 51–60 (2003)
Cong, Y. et al. Fast rotational matching of single-particle images. J. Struct. Biol. 152, 104–112 (2005)
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995)
Vranken, W. F. et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 (2005)
Bartels, C., Xia, T. H., Billeter, M., Guntert, P. & Wuthrich, K. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR 6, 1–10 (1995)
Kay, L. E., Ikura, M., Tschudin, R. & Bax, A. Three-dimensional triple resonance NMR spectroscopy of isotopically enriched proteins. J. Magn. Reson. 213, 423–441 (1990)
Salzmann, M., Wider, G., Pervushin, K. & Wuthrich, K. Improved sensitivity and coherence selection for [15N,1H]-TROSY elements in triple resonance experiments. J. Biomol. NMR 15, 181–184 (1999)
Szyperski, T., Neri, D., Leiting, B., Otting, G. & Wuthrich, K. Support of 1H NMR assignments in proteins by biosynthetically directed fractional 13C-labeling. J. Biomol. NMR 2, 323–334 (1992)
Schwieters, C. D., Kuszewski, J., Tjandra, N. & Clore, G. M. The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 160, 65–71 (2003)
Shen, Y., Delaglio, F., Cornilescu, G. & Bax, A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 44, 213–223 (2009)
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. W. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993)
Acknowledgements
We thank Y. Balazs for helping with ITC measurement and data analysis and Y. Zhang and M. Cao from the EM facility of NCPSS for their assistance with EM data collection. The NMR data were collected at the NMR facility of NCPSS and MIT-Harvard CMR (supported by NIH grant P41 EB-002026). This work was supported by CAS grant XDB08030301 and NIH grant GM094608 to J.J.C. V.K.M. is an Investigator of the Howard Hughes Medical Institute. Y.C. is supported by CAS grant XDB08030201. C.C. is supported by the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
T.C., Y.D., Y.S., C.C., V.K.M. and J.J.C. conceived the study; T.C., Y.S. and C.C. designed protein constructs for structural studies; C.C. and K.O. performed inhibitor binding studies; Y.S., A.L.M. and Z.G. performed structure guided functional experiments and analysis; Y.D., L.K. and Y.C. prepared EM samples and performed EM analysis. K.O., T.C., C.C. and J.J.C. collected NMR data and solved the structure; V.K.M., J.J.C. and K.O. wrote the paper and all authors contributed to editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Multiple sequence alignment of the full-length hMCU, full-length cMCU and cMCU-ΔNTD.
Residues that are invariant in all three sequences are shaded in red. Partially conserved and much less conserved residues are shaded in blue and grey, respectively. The mutations introduced in cMCU-ΔNTD are shaded in yellow. D and E in the DXXE motif are indicated by downward arrow. The serine residue shown to be involved in Ru360 inhibition is indicated by an asterisk4. Helical segments, as determined by NMR in this study, are indicated by cylinders and labelled as in the main text. The accession numbers for hMCU and cMCU are NM_138357.1 and NP_500892.1, respectively.
Extended Data Figure 2 Biochemical analysis of cMCU-ΔNTD oligomeric state.
a, Elution peak of cMCU-ΔNTD from Superdex 200 10/300 GL column in 20 mM MES, pH 6.4, 75 mM NaCl, 0.48 mM foscholine-14, 0.3 mM NaN3 and 2 mM EDTA. b, SDS–PAGE analysis of the elution peak showing sample purity >95%. c, SEC-MALS analysis of the diluted NMR sample of cMCU-ΔNTD. The SEC-MALS/UV/refractive index measurement was used to determine cMCU-ΔNTD molecular mass based on the three-detector method28. In this method, since the foscholine-14 detergent does not have UV absorption at 280 nm, the protein mass is directly calculated without correcting for the bound micelle. Chromatograms show the readings from the light scattering at 90° (red), refractive index (blue), and UV (green) detectors. The left and right axes represent the light scattering detector reading and molecular mass, respectively. The black curve represents the calculated molecular mass, and the average mass of the elution peak of cMCU-ΔNTD is 92 kDa. Note the ~10 ml difference in elution volumes between a and c is due to the volume of solution feeding from SEC to MALS. d, SDS–PAGE analysis of chemical crosslinking of the diluted cMCU-ΔNTD NMR sample. The reaction mixture contains 0.1 mM cMCU-ΔNTD (monomer), 3 mM foscholine-14, and various amounts of DTSSP. The reactions were quenched after 1 h by the addition of 2 μl of 1 M Tris, pH 7.5. The quenched samples were loaded to 12% Bis-Tris gel (Novex Life Technologies). The gel was silver stained using the standard protocol. The five lanes to the right of the molecular mass marker correspond to DTSSP concentrations of 0, 5, 7, 10 and 15 mM. The band that corresponds to a pentamer showed the most obvious increase in intensity as a function of [DTSSP].
Extended Data Figure 3 The cMCU-ΔNTD methyl group resonances with residue specific assignment.
The 1H–13C HSQC was recorded with 28 ms constant-time 13C evolution at 900 MHz.
Extended Data Figure 4 Single particle EM of the cMCU-ΔNTD oligomeric complex.
a, Typical image of the cMCU-ΔNTD oligomers negatively stained with uranyl formate. The bar corresponds to 50 nm in length. A selected subset of cMCU-ΔNTD particles is highlighted with white circles. Shown in the top left corner are a typical top/bottom view and a typical side view of the selected particles. b, Gallery of 100 out of 202 reference-free 2D class averages of the particles, which revealed the existence of five-fold symmetry in the complex (pentagon shapes marked by yellow circles). c, Comparison of different views of the 3D EM density map reconstructed without enforcing C5 symmetry (top) with the corresponding views of the map reconstituted with symmetry (bottom). The largest discrepancies are in the middle bulge region between the TM and CC domains, possibly due to the lack of rigid structure in the large L2 loop. d, Comparison of the 2D projections (top) from the 3D EM density map with the corresponding reference-free 2D classes. e, Estimation of resolution of the final 3D reconstruction. The Fourier shell correlation (FSC) suggests a resolution of ~18 Å using the 0.5 criterion.
Extended Data Figure 5 Intermonomer NOEs from mixed isotope labelled sample.
Examples are taken from the 3D 15N-edited NOESY-TROSY of the mixed labelled sample containing 1:1 mixture of (15N, 2H)-labelled cMCU-ΔNTD and (15% 13C)-labelled cMCU-ΔNTD. a, Sample 1H–1H strips at various 15N chemical shifts showing intermonomer NOEs between backbone amide proton and aliphatic protons for the C-terminal CCH domain. The NOE spectrum was recorded at 23 °C at 900 MHz. b, Sample strips showing intermonomer NOEs within the TMH2 pore as well as the selectivity filter region. The NOE spectrum was recorded at 33 °C at 900 MHz. On the right of each panel, intermonomer NOEs in the context of the structure are shown as red lines.
Extended Data Figure 6 Structural ensemble of the cMCU-ΔNTD pentamer derived from NMR restraints and fitting to the EM density map.
a, Ensemble of the 15 lowest-energy structures calculated using NMR-derived structural restraints (see Extended Data Table 1). The unstructured loops L1 (residues 166–179) and L2 (residues 272–292) are not shown for clarity. b, The NMR structure of cMCU-ΔNTD without the loop regions L1 and L2 was fitted to the EM volume using rigid body fitting (the ‘fit’ tool in Chimera). The L1 and L2 are however included for display. Top view from the intermembrane space side. c, Bottom view from the matrix side. d, e, Two different side views. Note that the loop regions appear disordered due to the lack of NMR-derived structural restraints. This does not necessarily mean that they do not acquire any stable conformation. The presumed detergent molecules around the membrane-embedded region are not taken into account in this fit.
Extended Data Figure 7 SDS–PAGE analysis of chemical crosslinking of the CCH peptide.
The reaction mixtures containing 0.1 mM peptide (cMCU residues 288–316 plus the C-terminal L and E as in the cMCU-ΔNTD construct) and various amounts of DTSSP were quenched after 1 h of reaction by the addition of 1 μl of 1 M Tris, pH 7.5. The quenched samples were loaded to 12% Bis-Tris gel (Novex Life Technologies). The gel was silver stained using the standard protocol. The four lanes to the right of the molecular mass marker correspond to DTSSP:CCH ratios of 0, 10:1, 30:1 and 50:1.
Extended Data Figure 8 Surface representation for revealing the surface-exposed and core amino acid properties of the cMCU-ΔNTD pentamer.
Hydrophobic, polar and charged residues are shown in yellow, cyan and blue, respectively. The hydrophobic residues include A, I, L, F, V, P and G, the polar residues include Q, N, H, S, T, Y, C, M and W, and the charged residues include K, R, D and E. The solid lines indicate the hydrophobic core boundaries of the presumed lipid bilayer. a, Pentamer with unstructured loops removed. b, The same view as in a but with the front subunit removed to reveal the core.
Extended Data Figure 9 Deletion of the NTD (amino acids 58–186) in HsMCU (HsMCU-ΔNTD) does not impair its function.
Representative traces of Ca2+ uptake in digitonin-permeabilized cells after addition of 50 μM CaCl2 are shown on the left. The bar graph shows the rate of Ca2+ uptake relative to wild-type HEK-293T cells (mean ± s.d., n = 4). Cell lysates were analysed by immunoblotting using an anti-Flag antibody to detect expression of MCU protein. ATP5A was used as loading control.
Rights and permissions
About this article
Cite this article
Oxenoid, K., Dong, Y., Cao, C. et al. Architecture of the mitochondrial calcium uniporter. Nature 533, 269–273 (2016). https://doi.org/10.1038/nature17656
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17656
This article is cited by
-
Reduced mitochondrial calcium uptake in macrophages is a major driver of inflammaging
Nature Aging (2023)
-
Experimental and computational studies of tautomerism pyridine carbonyl thiosemicarbazide derivatives
Structural Chemistry (2023)
-
The interplay of dopamine metabolism abnormalities and mitochondrial defects in the pathogenesis of schizophrenia
Translational Psychiatry (2022)
-
Mitochondrial calcium uniporter stabilization preserves energetic homeostasis during Complex I impairment
Nature Communications (2022)
-
Decreased Vascular Bundle 1 affects mitochondrial and plant development in rice
Rice (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.