Abstract
Methyl-CpG binding protein 2 (MeCP2) has crucial roles in transcriptional regulation and microRNA processing1,2,3,4. Mutations in the MECP2 gene are found in 90% of patients with Rett syndrome, a severe developmental disorder with autistic phenotypes5. Duplications of MECP2-containing genomic segments cause the MECP2 duplication syndrome, which shares core symptoms with autism spectrum disorders6. Although Mecp2-null mice recapitulate most developmental and behavioural defects seen in patients with Rett syndrome, it has been difficult to identify autism-like behaviours in the mouse model of MeCP2 overexpression7,8. Here we report that lentivirus-based transgenic cynomolgus monkeys (Macaca fascicularis) expressing human MeCP2 in the brain exhibit autism-like behaviours and show germline transmission of the transgene. Expression of the MECP2 transgene was confirmed by western blotting and immunostaining of brain tissues of transgenic monkeys. Genomic integration sites of the transgenes were characterized by a deep-sequencing-based method. As compared to wild-type monkeys, MECP2 transgenic monkeys exhibited a higher frequency of repetitive circular locomotion and increased stress responses, as measured by the threat-related anxiety and defensive test9. The transgenic monkeys showed less interaction with wild-type monkeys within the same group, and also a reduced interaction time when paired with other transgenic monkeys in social interaction tests. The cognitive functions of the transgenic monkeys were largely normal in the Wisconsin general test apparatus, although some showed signs of stereotypic cognitive behaviours. Notably, we succeeded in generating five F1 offspring of MECP2 transgenic monkeys by intracytoplasmic sperm injection with sperm from one F0 transgenic monkey, showing germline transmission and Mendelian segregation of several MECP2 transgenes in the F1 progeny. Moreover, F1 transgenic monkeys also showed reduced social interactions when tested in pairs, as compared to wild-type monkeys of similar age. Together, these results indicate the feasibility and reliability of using genetically engineered non-human primates to study brain disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Meehan, R. R., Lewis, J. D., McKay, S., Kleiner, E. L. & Bird, A. P. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell 58, 499–507 (1989)
Nan, X., Campoy, F. J. & Bird, A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88, 471–481 (1997)
Young, J. I. et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc. Natl Acad. Sci. USA 102, 17551–17558 (2005)
Cheng, T. L. et al. MeCP2 suppresses nuclear microRNA processing and dendritic growth by regulating the DGCR8/Drosha complex. Dev. Cell 28, 547–560 (2014)
Amir, R. E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet. 23, 185–188 (1999)
Ramocki, M. B. et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann. Neurol. 66, 771–782 (2009)
Samaco, R. C. et al. Crh and Oprm1 mediate anxiety-related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nature Genet. 44, 206–211 (2012)
Collins, A. L. et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 13, 2679–2689 (2004)
Kalin, N. H. & Shelton, S. E. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science 243, 1718–1721 (1989)
Nakagawa, T. et al. Generation of lentiviral transgenic rats expressing glutamate receptor interacting protein 1 (GRIP1) in brain, spinal cord and testis. J. Neurosci. Methods 152, 1–9 (2006)
Frye, R. E., Melnyk, S. & Macfabe, D. F. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl. Psychiatry 3, e220 (2013)
Yan, G. et al. Genome sequencing and comparison of two nonhuman primate animal models, the cynomolgus and Chinese rhesus macaques. Nature Biotechnol. 29, 1019–1023 (2011)
Higashino, A. et al. Whole-genome sequencing and analysis of the Malaysian cynomolgus macaque (Macaca fascicularis) genome. Genome Biol. 13, R58 (2012)
Huh, J. W. et al. Large-scale transcriptome sequencing and gene analyses in the crab-eating macaque (Macaca fascicularis) for biomedical research. BMC Genomics 13, 163 (2012)
Chahrour, M. et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320, 1224–1229 (2008)
Ramiro, L. S., Madrid, B. J. & Brown, D. W. Adverse childhood experiences (ACE) and health-risk behaviors among adults in a developing country setting. Child Abuse Negl. 34, 842–855 (2010)
Drago, L. & Thierry, B. Effects of six-day maternal separation on tonkean macaque infants. Primates 41, 137–145 (2000)
Suomi, S. J. Early determinants of behaviour: Evidence from primate studies. Br. Med. Bull. 53, 170–184 (1997)
Feng, X. L. et al. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc. Natl Acad. Sci. USA 108, 14312–14317 (2012)
Bauman, M. D., Lavenex, P., Mason, W. A., Capitanio, J. P. & Amaral, D. G. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J. Cogn. Neurosci. 16, 1388–1411 (2004)
Emery, N. J. et al. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 115, 515–544 (2001)
Golub, M. S., Hogrefe, C. E. & Germann, S. L. Iron deprivation during fetal development changes the behavior of juvenile rhesus monkeys. J. Nutr. 137, 979–984 (2007)
Makori, N., Watson, R. E., Hogrefe, C. E., Lalayeva, N. & Oneda, S. Object discrimination and reversal learning in infant and juvenile non-human primates in a non-clinical laboratory. J. Med. Primatol. 42, 147–157 (2013)
Sackett, G., Ruppenthal, G., Hewitson, L., Simerly, C. & Schatten, G. neonatal behavior and infant cognitive development in rhesus macaques produced by assisted reproductive technologies. Dev. Psychobiol. 48, 243–265 (2006)
Harlow, H. F. The development of learning in the rhesus monkey. Am. Sci. 47, 458–479 (1959)
Ha, J. C., Mandell, D. J. & Gray, J. Two-item discrimination and Hamilton search learning in infant pigtailed macaque monkeys. Behav. Processes 86, 1–6 (2011)
Sasaki, E. et al. Generation of transgenic non-human primates with germline transmission. Nature 459, 523–527 (2009)
Moran, S. et al. Germline transmission in transgenic Huntington’s disease monkeys. Theriogenology 84, 277–285 (2015)
Liu, H. et al. TALEN-mediated gene mutagenesis in rhesus and cynomolgus monkeys. Cell Stem Cell 14, 323–328 (2014)
Liu, Z. et al. Generation of a monkey with MECP2 mutations by TALEN-based gene targeting. Neurosci. Bull. 30, 381–386 (2014)
Sun, Q. et al. Efficient reproduction of cynomolgus monkey using pronuclear embryo transfer technique. Proc. Natl Acad. Sci. USA 105, 12956–12960 (2008)
Chan, A. W. & Yang, S. H. Generation of transgenic monkeys with human inherited genetic disease. Methods 49, 78–84 (2009)
Zheng, P., Bavister, B. D. & Ji, W. Z. Energy substrate requirement for in vitro maturation of oocytes from unstimulated adult rhesus monkeys. Mol. Reprod. Dev. 58, 348–355 (2001)
Zheng, P., Wang, H., Bavister, B. D. & Ji, W. Maturation of rhesus monkey oocytes in chemically defined culture media and their functional assessment by IVF and embryo development. Hum. Reprod. 16, 300–305 (2001)
Janovitz, T. et al. High-throughput sequencing reveals principles of adeno-associated virus serotype 2 integration. J. Virol. 87, 8559–8568 (2013)
Cohn, L. B. et al. HIV-1 integration landscape during latent and active infection. Cell 160, 420–432 (2015)
Xiao, J. et al. Rearrangement structure-independent strategy of CNV breakpoint analysis. Mol. Genet. Genomics 289, 755–763 (2014)
Du, R. et al. Efficient typing of copy number variations in a segmental duplication-mediated rearrangement hotspot using multiplex competitive amplification. J. Hum. Genet. 57, 545–551 (2012)
Kalin, N. H., Shelton, S. E., Davidson, R. J. & Kelley, A. E. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J. Neurosci. 21, 2067–2074 (2001)
Kalin, N. H., Shelton, S. E. & Davidson, R. J. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 24, 5506–5515 (2004)
Rogers, J., Shelton, S. E., Shelledy, W., Garcia, R. & Kalin, N. H. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes Brain Behav. 7, 463–469 (2008)
Wang, K. et al. MapSplice: accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 38, e178 (2010)
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010)
Liu, Z. et al. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res. 26, 139–142 (2015)
Acknowledgements
We thank M.-m. Poo for comments on the manuscript, Y.-Z. Li, Y.-Z. Lu, F. Liu and X. Zhou for maintaining monkey colony, W. Lu, X.-H. Guo and Y. F. Zhou of Fudan Children’s Hospital for assistance in mass spectrometry and electroencephalogram, D. Chen and B. Zhang of Novel Bioinformatics for RNA-seq data analysis, and C.-H. Li of Shanghai Geneskies Company for analysis of genomic integration sites. This work was supported by CAS Strategic Priority Research Program (XDB02050400), the MoST 973 Program (2011CBA00400), NSFC grants (91432111, 91232712 and 81527901), National Key Technology R&D Program of China 2014BAI03B00, Shanghai City Committee of Science and Technology Project 14140900100.
Author information
Authors and Affiliations
Contributions
Z.Q. and Q.S. conceived and supervised the project. T.-L.C. constructed the lentiviral constructs. Q.S. and Z.L. performed the cynomolgus oocytes preparation and injection. Y.-J.C., Y.W., C.-C.Z., Y.-H.N. and Z.L. contributed to monkey reproductive experiments. Y.-F.Z. performed PCR-based genotyping experiments. Z.-F.C., W.-J.B., X.-D.Z. and X.Y. performed immunohistochemistry and AccuCopy experiments. C.C., B.L., X.S. and Z.-Q.X. performed western blot experiments. X.L. and J.-J.W. performed behavioural analysis. J.-T.Z. and N.G. performed WGTA tests. W.-H.Z. and X.X. contributed to metabolic measurements and behavioural analysis. T.-L.C. and X.L. performed genomic integration sites analysis based on deep-sequencing. J.X., L.Z. and F.Z. helped with identification of genomic integration sites of transgenes. Z.Q. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
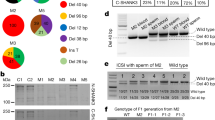
Extended Data Figure 1 Analysis of genomic integration sites of transgenes in F0 TG monkeys.
a, Genomic DNA were extracted from hair roots of WT and TG monkeys, digested and analysed by agarose gel. Radioactive probe labelled by 32P were prepared against the hMeCP2-2a-GFP transgene. Blots were transferred to membrane and hybridized with the probes. Images were acquired by exposing the blot to a phosphor-imager. Asterisk indicates target band. b, Flowchart of deep-sequencing-based methods for identifying genomic integration sites of lentiviral transgenes. Genomic integration sites of lentiviral transgenes are composed as three parts, endogenous genomic segments (black), LTRs (blue) and transgenes (red). Genomic DNA was sonicated, end-repaired and ligated to a Y-shaped adaptor, then subjected to two rounds of amplifications by the LTR1 + YP1 and LTR2 + YP2 primer sets. Illumina sequencing linkers were added onto segments and performed paired-end high-throughput sequencing. Target sequences containing LTR and endogenous genomic segments were collected and analysed. c, Comparison of copy numbers obtained from two methods among F0 TG monkeys. Red denotes copy numbers from AccuCopy (MECP2 and mCherry transgenes); blue denotes LTR insertion sites from deep-sequencing.
Extended Data Figure 2 Physical parameters measured for monkeys.
Developmental changes in the body weight of 8 TG and 18 WT monkeys. a–g, Body weight, abdominal circumference, heart rate, respiratory rate, head circumference, body temperature and head–truck length were measured for 8 MECP2 TG and 18 WT monkeys. *P < 0.05 (Mann–Whitney U test). Error bars denote s.e.m.
Extended Data Figure 3 Fatty acid measurements for TG and WT monkeys.
a, Blood samples collected at 18 months of age. b, Blood samples collected at 36 months of age. The blood levels of different forms of fatty acids were measured by mass spectrometry, with each bar represents results from three independent samples. C0, total fatty acid contents. All data are normalized to the average values of parallel blood samples from WT monkeys. *P < 0.05 (Student’s t-test). Error bars denote s.e.m.
Extended Data Figure 4 Physical growth parameters measured for monkey T05 and transcriptome analysis of MECP2 transgenic monkey.
a–c, Body weight, head circumference and body temperature were measured for monkey T05. *P < 0.05 (Mann–Whitney U test), together with the average data from all other TG and WT monkey monitored. The monkey T05 died at 20 months of age. d, Volcano map for alterations in gene expression in the TG monkeys (T14, T05, T07 and T09), as compared to four WT monkeys. Red dots denote genes with a >2-fold change (FC) in expression (log2(FC(TG/WT)) > 1 or <−1). Blue dots denote genes with no significant change in expression (P > 0.05). e, Heat map representation of the selected genes involved in metabolic pathways and brain development. Gene expression is coded in pseudocolour scale (−0.14 to 0.14). Red denotes higher expression in TG monkeys; green denotes lower expression in TG monkeys, as compared to WT monkeys. Error bars denote s.e.m.
Extended Data Figure 5 Anxiety responses in MECP2 TG monkeys.
a, Schematic illustration of the protocol of TAD test. b, Boxplots of the total numbers of grunt sounds made by WT and TG monkeys during the gaze period (‘step 4’ in the TAD test) at 36 months of age. c, Total grunts responses of wild-type and transgenic monkeys during TAD tests. d, Total vocal responses of wild-type and transgenic monkeys during TAD tests. *P < 0.05 (Student’s t-test). Ends of whiskers represent the minimum and maximum of data points. The line within box represents the median (odd numbers of data points) or second quartile (even number of data points). The bottom and top edge of the box represents the first and the third quartile, respectively.
Extended Data Figure 6 Spectrograms of typical sounds produced by the monkey in the TAD test.
a–c, The power at different frequencies (ordinate) is colour-coded (red denotes higher power). Three typical sounds, grunt (a), coo (b) and scream (c), are shown.
Extended Data Figure 7 Social interaction between monkeys from the same group (familiar pairing).
a–f, The average total time spent in sitting together during pairing in an isolated observation cage for each TG monkey (T04, T07, T08, T06, T09 and T11) with either a WT or a TG monkey was presented individually for six TG monkeys tested. T04 (a), T07 (b), T08 (c), T06 (d), T09 (e) and T11 (f). (See Supplementary Table 4c for grouping.) Each observation lasted 60 min daily for 5 days.
Extended Data Figure 8 Schematic illustration of experimental procedures of WGTA test.
a, Black/white test. b, Boxplots of days required to pass the adaptation, discrimination and reversal steps in the black/white test for six WT and eight TG monkeys. Ends of whiskers represent the minimum and maximum of data points. The line within box represents the median (odd numbers of data points) or second quartile (even number of data points). The bottom and top edge of the box represents the first and the third quartile, respectively. c, Hamilton search test. d, Learning curves for the Hamilton forced set-breaking test after passing the black/white test (for six WT and seven TG monkeys). The difference between the two groups was at a significance level of P = 0.06 (Mann–Whitney U test). Error bars denote s.e.m.
Extended Data Figure 9 Performance of WT and TG monkeys in learning set of WGTA test.
a, Learning set test. Correct rate of monkeys in the reward-shape association learning test plotted individually against trials, with data points represents average correct rates over 180 trials. b, WT monkeys. c, TG monkeys.
Supplementary information
Supplementary Figures
This file contains the Western blots raw data for Figures 1d, e, f, h and 4c. (PDF 3486 kb)
Supplementary Tables
This file contains Supplementary Tables 1-7 (PDF 428 kb)
Supplementary Information
This zipped file contains Supplementary Audio files 1-3 comprising: (1) Grunt vocal responses of monkeys examined in TAD tests; (2) Coo vocal responses of monkeys examined in TAD tests; (3) Stream vocal responses of monkeys examined in TAD tests. (ZIP 686 kb)
Video 1:Example of normal motor behaviours
This video shows the representative normal motor behaviours for monkeys we examined. (AVI 3223 kb)
Video 2: Example of repetitive motor behaviours
This video shows the typical repetitive motor behaviours for four F0 TG monkeys out of eight F0 TG monkeys we examined. (AVI 3223 kb)
Video 3: Example of social interaction behaviours.
This video shows the representative social interaction behaviours of monkeys, such as sitting together, in natural community. (AVI 4353 kb)
Video 4: Social Interaction behaviours in pairing experiments
This video shows the typical social behaviour, sitting together, in pairing experiments. (AVI 393 kb)
Video 5: Examples of learning behaviors of monkey in learning set of WGTA tests.
This video shows the example of learned behaviours for W09 out of five WT monkeys we examined. (AVI 2439 kb)
Video 6. Examples of stereotypic behaviours of MECP2 F0 TG monkey in learning set of WGTA tests
This video shows the example of stereotypic responses of F0 TG monkeys (T09) out of seven F0 TG monkeys we examined. (AVI 2374 kb)
Rights and permissions
About this article
Cite this article
Liu, Z., Li, X., Zhang, JT. et al. Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature 530, 98–102 (2016). https://doi.org/10.1038/nature16533
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature16533
This article is cited by
-
Rhesus infant nervous temperament predicts peri-adolescent central amygdala metabolism & behavioral inhibition measured by a machine-learning approach
Translational Psychiatry (2024)
-
Genetics of human brain development
Nature Reviews Genetics (2024)
-
Nexus between genome-wide copy number variations and autism spectrum disorder in Northeast Han Chinese population
BMC Psychiatry (2023)
-
Modeling SHANK3-associated autism spectrum disorder in Beagle dogs via CRISPR/Cas9 gene editing
Molecular Psychiatry (2023)
-
Social isolation and the brain: effects and mechanisms
Molecular Psychiatry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.