Abstract
Many procedures in modern clinical medicine rely on the use of electronic implants in treating conditions that range from acute coronary events to traumatic injury1,2. However, standard permanent electronic hardware acts as a nidus for infection: bacteria form biofilms along percutaneous wires, or seed haematogenously, with the potential to migrate within the body and to provoke immune-mediated pathological tissue reactions3,4. The associated surgical retrieval procedures, meanwhile, subject patients to the distress associated with re-operation and expose them to additional complications5,6,7,8. Here, we report materials, device architectures, integration strategies, and in vivo demonstrations in rats of implantable, multifunctional silicon sensors for the brain, for which all of the constituent materials naturally resorb via hydrolysis and/or metabolic action9,10,11,12, eliminating the need for extraction. Continuous monitoring of intracranial pressure and temperature illustrates functionality essential to the treatment of traumatic brain injury2,13; the measurement performance of our resorbable devices compares favourably with that of non-resorbable clinical standards. In our experiments, insulated percutaneous wires connect to an externally mounted, miniaturized wireless potentiostat for data transmission. In a separate set-up, we connect a sensor to an implanted (but only partially resorbable) data-communication system, proving the principle that there is no need for any percutaneous wiring. The devices can be adapted to sense fluid flow, motion, pH or thermal characteristics, in formats that are compatible with the body’s abdomen and extremities, as well as the deep brain, suggesting that the sensors might meet many needs in clinical medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Poole, J. E. Present guidelines for device implantation: clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy. Circulation 129, 383–394 (2014)
Brain, T. F. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J. Neurotrauma 24, S37–S44 (2007)
Chamis, A. L. et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 104, 1029–1033 (2001)
Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Rev. Microbiol. 2, 95–108 (2004)
Maytin, M. & Epstein, L. M. Lead extraction is preferred for lead revisions and system upgrades. Circ. Arrhythm. Electrophysiol. 3, 413–424 (2010)
Boutry, C. M. et al. Towards biodegradable wireless implants. Phil. Trans. R. Soc. A 370, 2418–2432 (2012)
Ott, K. et al. Retained intracranial metallic foreign bodies: report of two cases. J. Neurosurg. 44, 80–83 (1976)
Vajramani, G. V. et al. Persistent and intractable ventriculitis due to retained ventricular catheters. Br. J. Neurosurg. 19, 496–501 (2005)
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012)
Irimia-Vladu, M. “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 43, 588–610 (2014)
Bettinger, C. J. & Bao, Z. Organic thin film transistors fabricated on resorbable biomaterial substrates. Adv. Mater. 22, 651–655 (2010)
Luo, M., Martinez, A. W., Song, C., Herrault, F. & Allen, M. G. A microfabricated wireless RF pressure sensor made completely of biodegradable materials. J. Microelectromech. Syst. 23, 4–13 (2014)
Brogan, M. E. & Manno, E. M. Treatment of malignant brain edema and increased intracranial pressure after stroke. Curr. Treat. Options Neurol. 17, 327 (2015)
Liu, C. Foundations of MEMS Ch. 6 (Prentice Hall, 2011)
Moseley, P. T. & Crocker, J. Sensor Materials Ch. 4 (Institute of Physics Publishing, 1994)
Chang, H. et al. DNA-mediated fluctuations in ionic current through silicon oxide nanopore channels. Nano Lett. 4, 1551–1556 (2004)
Zheng, G., Patolsky, F., Cui, Y., Wang, W. U. & Lieber, C. M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nature Biotechnol. 23, 1294–1301 (2005)
Stern, E. et al. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 445, 519–522 (2007)
Gentile, P., Chiono, V., Carmagnola, I. & Hatton, P. V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 15, 3640–3659 (2014)
Hwang, S.-W. et al. Dissolution chemistry and biocompatibility of single-crystalline silicon nanomembranes and associated materials for transient electronics. ACS Nano 8, 5843–5851 (2014)
Kang, S.-K. et al. Dissolution behaviors and applications of silicon oxides and nitrides in transient electronics. Adv. Funct. Mater. 24, 4427–4434 (2014)
Yin, L. et al. Dissolvable metals for transient electronics. Adv. Funct. Mater. 24, 645–658 (2014)
Uslaner, J. M. et al. T-type calcium channel antagonism produces antipsychotic-like effects and reduces stimulant-induced glutamate release in the nucleus accumbens of rats. Neuropharmacol. 62, 1413–1421 (2012)
Barth, K. N. M., Onesti, S. T., Krauss, W. E. & Solomon, R. A. A simple and reliable technique to monitor intracranial pressure in the rat: technical note. Neurosurgery 30, 138–140 (1992)
Haure, P., Cold, G. E., Hansen, T. M. & Larsen, J. R. The ICP-lowering effect of 10° reverse Trendelenburg position during craniotomy is stable during a 10-minute period. J. Neurosurg. Anesthesiol. 15, 297–301 (2003)
Morgan, P. W. Structure and moisture permeability of film-forming polymers. Ind. Eng. Chem. 45, 2296–2306 (1953)
Meldrum, D. R. et al. Prospective characterization and selective management of the abdominal compartment syndrome. Am. J. Surg. 174, 667–673 (1997)
Olson, S. A. & Glasgow, R. R. Acute compartment syndrome in lower extremity musculoskeletal trauma. J. Am. Acad. Orthop. Surg. 13, 436–444 (2005)
Stiefel, M. F. et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J. Neurosurg. 103, 805–811 (2005)
Timofeev, I. et al. Extracellular brain pH with or without hypoxia is a marker of profound metabolic derangement and increased mortality after traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 422–427 (2013)
Suehiro, E. et al. Diverse effects of hypothermia therapy in patients with severe traumatic brain injury based on the computed tomography classification of the traumatic coma data bank. J. Neurotrauma 32, 353–358 (2015)
Mittal, R. et al. Use of bio-resorbable implants for stabilization of distal radius fractures: the United Kingdom patients’ perspective. Injury 36, 333–338 (2005)
Ye, T. et al. Management of grade III open dislocated ankle fractures: combined internal fixation with bioabsorbable screws/rods and external fixation. J. Am. Podiatr. Med. Assoc. 101, 307–315 (2011)
Yin, L., Bozler, C., Harburg, D. V., Omenetto, F. & Rogers, J. A. Materials and fabrication sequences for water soluble silicon integrated circuits at the 90 nm node. Appl. Phys. Lett. 106, 014105 (2015)
Huang, X. et al. Biodegradable materials for multilayer transient printed circuit boards. Adv. Mater. 26, 7371–7377 (2014)
Acknowledgements
S.-K.K. and co-workers are funded by the Defense Advanced Research Projects Agency. J.G.M. is supported by the National Institute of Mental Health, grant F31MH101956. The authors thank M. R. Bruchas at Washington University School of Medicine for providing immunohistochemistry facilities; M. R. MacEwan at Washington University School of Medicine for discussions on animal protocols; A. Manocchi at Transient Electronics Inc. for performing the dissolution test of polyanhydride; and H. Ning at Xerion Advanced Battery Corporation for assistance with running the BET measurements. H.C. was a Howard Hughes Medical Institute International Student Research Fellow. S.-W.H. was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant NRF-2015R1C1A1A02037560). G.P. and K.M.L. were supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (grants NRF-2007-00107 and NRF-2013M3A9D3045719).
Author information
Authors and Affiliations
Contributions
S.-K.K., S.-W.H., D.V.H., N.A.K., S.Y., J.S., H.Y., R.C.W., C.H.L., S.C., D.S.W., J.C., P.V.B. and J.A.R. designed and fabricated the sensors and interfaces. S.-K.K., S.M.L., J.S., J.K. S.H.L. and J.A.R. designed, fabricated and analysed the near-field communication system with the sensor. S.-K.K., R.K.J.M., S.M.L., D.V.H., H.C., P.G., S.Y., J.S., M.S., R.C.W., C.H.L., B.V., Z.L., Y.H., W.Z.R. and J.A.R. conceived the idea and performed the experiments and analysis. R.K.J.M., P.G., J.G.M., M.S., G.P., A.D.G., A.H.K., K.-M.L. and W.Z.R. analysed the immunohistochemistry. S.-K.K., R.K.J.M., S.-W.H., S.M.L., D.V.H., H.C., W.Z.R. and J.A.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
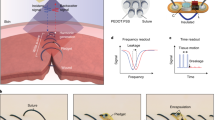
Extended Data Figure 1 Fully implantable near-field communication (NFC) system with bioresorbable interface and intracranial sensors.
a, Diagram of a fully implantable NFC system. This device uses a magnesium foil (~50 μm) for the inductive coil, interconnects and electrodes; patterned silicon nanomembranes (Si-NMs, ~300 nm) for resistors; conventional capacitors; and an advanced NFC microchip for data acquisition, processing and transmission. PLGA serves as the substrate and for encapsulation. The diameter of the entire device is about 15 mm. b, Image of this type of NFC system integrated with a bioresorbable pressure sensor. c, Diagram of the operational principles. d, Series of images showing accelerated dissolution of the NFC system inserted into an ACSF at 60 °C. e, Diagram of the implantation process. The bioresorbable sensors reside in the intracranial space, while the NFC system is located extracranially, on the outside surface of the skull, beneath the skin. Bioresorbable, thin metal wires interconnect the NFC system and the sensors. f, Real-time wireless measurements of ICP, showing transient increases induced by the Valsalva manoeuvre (red, data obtained from a transient ICP sensor; blue, data obtained from a commercial ICP sensor). g, Increase in ICT owing to application of a heating blanket around the head, as determined by bioresorbable (red) and commercial (blue) sensors. h, Demonstrations of implantation and suturing in a rat model. A biodegradable surgical glue (TISSEAL) seals the intracranial space.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Discussion, Supplementary References, Supplementary Table 1 and Supplementary Figures 1-39. (PDF 7154 kb)
Rights and permissions
About this article
Cite this article
Kang, SK., Murphy, R., Hwang, SW. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016). https://doi.org/10.1038/nature16492
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature16492
This article is cited by
-
Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation
Nature Communications (2024)
-
Stretchable liquid metal based biomedical devices
npj Flexible Electronics (2024)
-
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring
Nano-Micro Letters (2024)
-
Biodegradable electronics: a two-decade bibliometric analysis
Quality & Quantity (2024)
-
Intracranial pressure monitoring in neurosurgery: the present situation and prospects
Chinese Neurosurgical Journal (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.