Abstract
How the brain selects appropriate sensory inputs and suppresses distractors is unknown. Given the well-established role of the prefrontal cortex (PFC) in executive function1, its interactions with sensory cortical areas during attention have been hypothesized to control sensory selection2,3,4,5. To test this idea and, more generally, dissect the circuits underlying sensory selection, we developed a cross-modal divided-attention task in mice that allowed genetic access to this cognitive process. By optogenetically perturbing PFC function in a temporally precise window, the ability of mice to select appropriately between conflicting visual and auditory stimuli was diminished. Equivalent sensory thalamocortical manipulations showed that behaviour was causally dependent on PFC interactions with the sensory thalamus, not sensory cortex. Consistent with this notion, we found neurons of the visual thalamic reticular nucleus (visTRN) to exhibit PFC-dependent changes in firing rate predictive of the modality selected. visTRN activity was causal to performance as confirmed by bidirectional optogenetic manipulations of this subnetwork. Using a combination of electrophysiology and intracellular chloride photometry, we demonstrated that visTRN dynamically controls visual thalamic gain through feedforward inhibition. Our experiments introduce a new subcortical model of sensory selection, in which the PFC biases thalamic reticular subnetworks to control thalamic sensory gain, selecting appropriate inputs for further processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001)
Buschman, T. J. & Miller, E. K. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315, 1860–1862 (2007)
Fritz, J., Shamma, S., Elhilali, M. & Klein, D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nature Neurosci. 6, 1216–1223 (2003)
Rodgers, C. C. & DeWeese, M. R. Neural correlates of task switching in prefrontal cortex and primary auditory cortex in a novel stimulus selection task for rodents. Neuron 82, 1157–1170 (2014)
Zhang, S. et al. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665 (2014)
Glickfeld, L. L., Histed, M. H. & Maunsell, J. H. Mouse primary visual cortex is used to detect both orientation and contrast changes. J. Neurosci. 33, 19416–19422 (2013)
Hubel, D. H. & Wiesel, T. N. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. (Lond.) 160, 106–154 (1962)
Newsome, W. T., Britten, K. H. & Movshon, J. A. Neuronal correlates of a perceptual decision. Nature 341, 52–54 (1989)
Hoover, W. B. & Vertes, R. P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 212, 149–179 (2007)
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011)
Halassa, M. M. et al. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nature Neurosci. 14, 1118–1120 (2011)
Zhao, S. et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nature Methods 8, 745–752 (2011)
Fritz, J. B., David, S. V., Radtke-Schuller, S., Yin, P. & Shamma, S. A. Adaptive, behaviorally gated, persistent encoding of task-relevant auditory information in ferret frontal cortex. Nature Neurosci. 13, 1011–1019 (2010)
Letzkus, J. J. et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 (2011)
McAlonan, K., Cavanaugh, J. & Wurtz, R. H. Guarding the gateway to cortex with attention in visual thalamus. Nature 456, 391–394 (2008)
Purushothaman, G., Marion, R., Li, K. & Casagrande, V. A. Gating and control of primary visual cortex by pulvinar. Nature Neurosci. 15, 905–912 (2012)
Saalmann, Y. B., Pinsk, M. A., Wang, L., Li, X. & Kastner, S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337, 753–756 (2012)
Pinault, D. The thalamic reticular nucleus: structure, function and concept. Brain Res. Brain Res. Rev. 46, 1–31 (2004)
Crick, F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc. Natl Acad. Sci. USA 81, 4586–4590 (1984)
Halassa, M. M. et al. State-dependent architecture of thalamic reticular subnetworks. Cell 158, 808–821 (2014)
O’Connor, D. H., Fukui, M. M., Pinsk, M. A. & Kastner, S. Attention modulates responses in the human lateral geniculate nucleus. Nature Neurosci. 5, 1203–1209 (2002)
Chen, C. & Regehr, W. G. Presynaptic modulation of the retinogeniculate synapse. J. Neurosci. 23, 3130–3135 (2003)
Cox, C. L., Huguenard, J. R. & Prince, D. A. Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proc. Natl Acad. Sci. USA 94, 8854–8859 (1997)
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014)
Grimley, J. S. et al. Visualization of synaptic inhibition with an optogenetic sensor developed by cell-free protein engineering automation. J. Neurosci. 33, 16297–16309 (2013)
Cremers, T. & Ebert, B. Plasma and CNS concentrations of Gaboxadol in rats following subcutaneous administration. Eur. J. Pharmacol. 562, 47–52 (2007)
Casagrande, V. A., Sáry, G., Royal, D. & Ruiz, O. On the impact of attention and motor planning on the lateral geniculate nucleus. Prog. Brain Res. 149, 11–29 (2005)
Mitchell, A. S. et al. Advances in understanding mechanisms of thalamic relays in cognition and behavior. J. Neurosci. 34, 15340–15346 (2014)
Ress, D. & Heeger, D. J. Neuronal correlates of perception in early visual cortex. Nature Neurosci. 6, 414–420 (2003)
Levitan, C. A., Ban, Y. H., Stiles, N. R. & Shimojo, S. Rate perception adapts across the senses: evidence for a unified timing mechanism. Sci. Rep. 5, 8857 (2015)
Mareschal, I., Calder, A. J., Dadds, M. R. & Clifford, C. W. Gaze categorization under uncertainty: psychophysics and modeling. J. Vis. 13, 18 (2013)
Hastie, T., Tibshirani, R. & Friedman, J. The Elements of Statistical Learning: Data Mining, Inference and Prediction 2nd edn, Ch. 7 (Springer, 2009)
Wallace, D. J. et al. Rats maintain an overhead binocular field at the expense of constant fusion. Nature 498, 65–69 (2013)
Brunetti, P. M. et al. Design and fabrication of ultralight weight, adjustable multi-electrode probes for electrophysiological recordings in mice. J. Vis. Exp. 91, e51675 (2014)
Fries, P., Neuenschwander, S., Engel, A. K., Goebel, R. & Singer, W. Rapid feature selective neuronal synchronization through correlated latency shifting. Nature Neurosci. 4, 194–200 (2001)
Szucs, A. Applications of the spike density function in analysis of neuronal firing patterns. J. Neurosci. Methods 81, 159–167 (1998)
Piscopo, D. M., El-Danaf, R. N., Huberman, A. D. & Niell, C. M. Diverse visual features encoded in mouse lateral geniculate nucleus. J. Neurosci. 33, 4642–4656 (2013)
De Araujo, I. E. et al. Neural ensemble coding of satiety states. Neuron 51, 483–494 (2006)
Ridder III, W. H. & Nusinowitz, S. The visual evoked potential in the mouse–origins and response characteristics. Vision Res. 46, 902–913 (2006)
Izaki, Y., Fujiwara, S. E. & Akema, T. Rat prefrontal response and prestimulation local field potential power in vivo. Neuroreport 19, 255–258 (2008)
Acknowledgements
We thank J. A. Movshon, W. Ma, R. W. Tsien, G. Fishell and D. Rinberg for helpful comments on the manuscript and G. J. Augustine for providing us with the SuperClomeleon construct and for helpful discussion around its use. The work was supported by the Swiss National Science Foundation (P2LAP3 151786) to R.D.W. and the Simons Foundation, the Sloan Foundation, the Brain and Behavior Research Foundation and the US National Institutes of Health (R00 NS078115) to M.M.H; M.M.H. is additionally supported by the Feldstein Medical Foundation, a Klingenstein-Simons Fellowship and a Biobehavioral Research Award for Innovative New Scientists (BRAINS) R01 (R01 MH107680) from the National Institute of Mental Health.
Author information
Authors and Affiliations
Contributions
M.M.H. conceived and designed all aspects of the study. R.D.W. devised the training paradigm for the cross-modal task and L.I.S. performed all associated programming. R.D.W. collected electrophysiological data. T.J.D. provided fibre photometry training, advice and rig designs; L.I.S. extended the method to FRET-based photometry, built the rig and collected data. R.D.W. analysed behavioural data and L.I.S. analysed psychophysical, electrophysiological and photometry data. M.N. generated the retrograde lentiviruses in-house, performed SuperClomeleon cloning into an AAV backbone and acquired confocal images. K.D. provided support for fibre photometry training. M.M.H. supervised the experiment, directed the analysis and wrote the manuscript. All authors read the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Cross-modal task training and performance validation.
Quantification of performance across training stages for the cross-modal task. The trial sequence for each training stage is indicated on the left. Improved performance was observed in the last three days of training relative to the first three for each stage. Bar graphs on the left (column 1) show the reduction in the error fraction (n = 15 mice,*P < 0.05, Wilcoxon rank-sum test), column 2 shows the number of consecutive correct responses (P-values shown, Kolmogorov–Smirnov test) and bar graphs on the right (column 3) show the probability of a correct response following a modality shift (*P < 0.05, Wilcoxon rank-sum test).
Extended Data Figure 2 Effects of cross-modal divided attention in the mouse.
Top row, single-mouse examples of visual detection performance during cross-modal divided attention and reversal learning. Comparison of performance under visual-only (black) and cross-modal (green) conditions are shown on the left. Although neither condition contained sensory conflict, the mere expectation of one increased detection threshold (≥124 trials per condition). Detection threshold was not affected by the presence of an auditory distractor during reversal learning (≥90 trials per condition), as shown on the right. Middle and bottom rows, group data normalized to peak performance (lapse rate), showing that the effects of divided attention on detection threshold were persistent. Bootstrap estimation of visual detection threshold shows a similar pattern as data in Fig. 1 (error bars are 95% confidence intervals).
Extended Data Figure 3 Comparable performance on trial types and intact overall auditory performance despite auditory stimuli being eliminated on a subset of ‘attend to vision’ trials.
Left, performance was comparable on auditory and maximum-intensity visual trials (n = 4 mice, same as in Fig. 1d). Right, mice exhibited comparable overall performance when auditory stimuli were eliminated from a subset of ‘attend to vision’ trials.
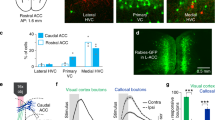
Extended Data Figure 4 Region- and timing-specific effects of optogenetic manipulation on cross-modal task performance.
a, Optogenetic disruption of auditory cortex during target stimulus anticipation disrupted performance specifically for auditory trials (n = 4 mice, **P < 0.01, Wilcoxon rank-sum test). Disruption of AAC (b) or lateral OFC (c) in VGAT-ChR2 mice or following localized injection of a ChR2-expressing virus did not affect performance (n = 4 mice (2 VGAT-ChR2 and 2 VGAT-Cre), 4 sessions per manipulation). d, In contrast, inactivation of prelimbic (PL) cortex led to robust reduction in performance in both types of manipulation (n = 8 mice (4 VGAT-ChR2 and 4 VGAT-Cre), *P < 0.05 Wilcoxon rank-sum test). e–h, Photobleaching experiment to quantify the spread of laser light. A coronal section (e) shows GFP bleaching following two-hour exposure to laser stimulation (6 mW, 50 Hz, 90% duty cycle). f–h, Fluorescence intensity quantification shows that the extent of light spread is limited to 300 μm around the tip of the optic fibre (n = 3 mice). Ant, anterior; FrA, frontal association cortex; lat, lateral; LO, lateral orbitofrontal cortex; med, medial; MO, medial orbitofrontal cortex; post, posterior; Stim, stimulation; Sham, sham surgery control; VO, ventral orbitofrontal cortex. Scale bar in e, 200 µm.
Extended Data Figure 5 Independently adjustable, multi-electrode recording of visTRN neurons.
a, b, Injection of DIO-ChR2-eYFP retrograde lentivirus into LGN labels visTRN neurons but not LGN interneurons. a, The histological image is the maximal projection of four 2-μm confocal planes showing labelling of visTRN neurons (inset shows a zoom view of cell bodies) approximately 1.34 mm posterior to Bregma. b, Image as in a, but from LGN of the same animal, approximately 2.46 mm posterior to Bregma (inset shows a zoom view of terminals). c, Schematic of independently adjustable multi-electrode drive. d, An example of activity recorded from different depths during adjustment. Distinct patterns of physiological activity are observed along the trajectory in the broadband local field potential signal (0.1 Hz–32 kHz). The numbers correspond to different recording sites (marked by the red dots on the schematic in c). e, High-pass-filtered signals (600 Hz–10 kHz) showing spiking activity, with isolated clustered units showing distinguishable waveform characteristics in distinct structures. f, Example peri-event time histograms of ChR2-mediated visTRN response. Top, response to laser activation (473 nm, ˜4 mW, stimulation, 20 ms). Bottom, response to visual stimuli (10-ms pulse). Blue and orange blocks indicate laser and visual stimulation, respectively.
Extended Data Figure 6 Distinct changes in visTRN firing rate during natural errors compared to errors due to PFC disruption.
a–c, Scatter plots showing the change in absolute firing rate for visTRN neurons for correct (a), incorrect (b) or disrupted-PFC trials (c). Insets show the cumulative probability plot of separation from the unity line (no change). Although correct trials had a lower firing rate in ‘attend to vision’ than in ‘attend to audition’ trials (n = 138, P < 0.001 Wilcoxon signed-rank test), this pattern was reversed for incorrect trials (n = 138, P < 0.05, Wilcoxon signed-rank test); this suggests that perhaps the animal was attending to the wrong modality. This reversal was not observed in trials with PFC disruption (despite mouse performance being at chance level).
Extended Data Figure 7 The effect of PFC disruption on visTRN activity is distinct from naturally occurring errors.
a, Scatter plots of response from visTRN neurons, comparing the modulation of their firing rate (change from baseline) under the two distinct anticipatory conditions. Each sample is a single cell. Colours denote significance reached for each cell on a trial-by-trial basis (red, visual; blue, auditory; purple, both; rank-sum-test comparison to baseline). Note that in correct performance (n = 138, 4 mice, P < 0.005, Wilcoxon signed-rank test), ‘attend to vision’ resulted in a negative shift and ‘attend to audition’ resulted in a positive shift, consistent with examples shown in Fig. 3. During naturally occurring error trials, the modulation is partially reversed for both trial types, suggesting that at least a subset of errors are the result of attending to the wrong modality. In contrast, PFC disruption (n = 56 cells, 2 mice) resulted in a weaker, non-uniform effect (‘attend to visual’ trials are less affected). b, Quantification of effects seen in a. N.S., not significant.
Extended Data Figure 8 The magnitude of behavioural disruption co-varies with the strength of optogenetic manipulation of the LGN or visTRN.
Activation of inhibitory terminals in the LGN with a 90% duty cycle laser (Fig. 2) resulted in maximal disruption of cross-modal performance. Activating visually labelled TRN with identical stimulation parameters resulted in a quantitatively lower behavioural effect. Reducing the duty cycle of visTRN stimulation to 10% resulted in no effect on accuracy, as shown previously4.
Extended Data Figure 9 Attentional modulation by LGN is not observed on error trials.
a, b, No significant difference was observed in the average firing rate of LGN neurons during stimulus anticipation (P = 0.63, Wilcoxon signed-rank test, n = 161 cells, 4 mice) or presentation (P = 0.74, Wilcoxon signed-rank test, n = 161) among trial types when behavioural outcomes were incorrect. c, Similar effects were observed for VEPs (visual, n = 324 trials; auditory, n = 302 trials; 4 mice).
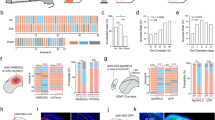
Extended Data Figure 10 Light-evoked fast transients from chloride photometry measured in the LGN are GABAA-receptor dependent and sensitive to visTRN and prelimbic inactivation in the cross-modal task.
a, Peak SuperClomeleon FRET- and YFP-control responses to light stimuli (50 ms, 0.1 Hz) delivered to the eye contralateral to the recorded LGN (n > 90 trials from 3 mice for SuperClomeleon and from 4 mice for YFP, ***P < 0.001, Friedman test). b, Chloride photometry transients are sensitive to the GABAA receptor antagonist flumazenil in a dose-dependent manner. Left, intraperitoneal injection of 15 mg kg–1 flumazenil resulted in a 90% peak reduction of light-evoked chloride photometry responses, which recovered over the course of 90–100 min as predicted by flumazenil pharmacokinetics. Insets show example traces of single events recorded during baseline, peak suppression and recovery. Right, quantification of the maximal suppressive effects and recovery of 5 mg kg–1 and 15 mg kg–1 flumazenil on chloride photometry responses (n> 90 trials from 3 mice, *P < 0.05, **P < 0.01, Friedman test). c, Cumulative distributions of unitary visual-evoked SuperClomeleon FRET peaks in response to light stimuli in the cross-modal task. Under baseline conditions, ‘attend to audition’ trials exhibited significantly larger amplitudes than ‘attend to vision’ trials, consistent with average data in Fig. 5f. Optogenetic silencing of visTRN neurons eliminated the difference between trial types and resulted in peak amplitudes comparable to baseline ‘attend to vision’ trials (n = 3 mice, P < 0.005 for ‘attend to audition’ trials vs all other trial types, Kolmogorov–Smirnov statistics with Bonferroni correction). d, Combined optogenetic and chloride photometry inactivation of different frontal cortical regions in the LGN while mice performed the cross-modal task. Only PL inactivation eliminates differential inhibition between visual and auditory trials (n = 6 mice, ***P < 0.001, Wilcoxon rank-sum test).
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion and additional references. (PDF 322 kb)
Example trials of cross-modal performance
Three trials are shown; the first is ‘attend to vision’ (left selection) the second is ‘attend to audition’ (left selection), and the third is ‘attend to vision’ (right selection). All trials are shown in normal speed and in slow-motion. The video illustrates the mechanics of the task and the impact of context (cueing) on selection. (MP4 26582 kb)
Rights and permissions
About this article
Cite this article
Wimmer, R., Schmitt, L., Davidson, T. et al. Thalamic control of sensory selection in divided attention. Nature 526, 705–709 (2015). https://doi.org/10.1038/nature15398
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature15398
This article is cited by
-
Gene expression networks regulated by human personality
Molecular Psychiatry (2024)
-
Functional plasticity of glutamatergic neurons of medullary reticular nuclei after spinal cord injury in mice
Nature Communications (2024)
-
Lifespan development of thalamic nuclei and characterizing thalamic nuclei abnormalities in schizophrenia using normative modeling
Neuropsychopharmacology (2024)
-
A Thalamocortical Perspective on Sleep Spindle Alterations in Neurodevelopmental Disorders
Current Sleep Medicine Reports (2024)
-
A distributed and efficient population code of mixed selectivity neurons for flexible navigation decisions
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.