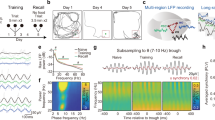
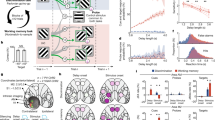
Abstract
Spatial working memory, the caching of behaviourally relevant spatial cues on a timescale of seconds, is a fundamental constituent of cognition. Although the prefrontal cortex and hippocampus are known to contribute jointly to successful spatial working memory, the anatomical pathway and temporal window for the interaction of these structures critical to spatial working memory has not yet been established. Here we find that direct hippocampal–prefrontal afferents are critical for encoding, but not for maintenance or retrieval, of spatial cues in mice. These cues are represented by the activity of individual prefrontal units in a manner that is dependent on hippocampal input only during the cue-encoding phase of a spatial working memory task. Successful encoding of these cues appears to be mediated by gamma-frequency synchrony between the two structures. These findings indicate a critical role for the direct hippocampal–prefrontal afferent pathway in the continuous updating of task-related spatial information during spatial working memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Baddeley, A. & Hitch, G. in Recent Advances in Learning and Motivation Vol. 8 (ed. Bower, G. A. ) 47–90 (Academic, 1974)
Klauer, K. C. & Zhao, Z. Double dissociations in visual and spatial short-term memory. J. Exp. Psychol. Gen. 133, 355–381 (2004)
Andrade, J. Working Memory in Perspective (Psychology Press, 2001)
Miyake, A. & Shah, P. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control (Cambridge Univ. Press, 1999)
Baddeley, A. Working memory: looking back and looking forward. Nature Rev. Neurosci. 4, 829–839 (2003)
de Zubicaray, G. I., McMahon, K., Wilson, S. J. & Muthiah, S. Brain activity during the encoding, retention, and retrieval of stimulus representations. Learn. Mem. 8, 243–251 (2001)
Curtis, C. E. & D’Esposito, M. The effects of prefrontal lesions on working memory performance and theory. Cogn. Affect. Behav. Neurosci. 4, 528–539 (2004)
Hyman, J. M., Zilli, E. A., Paley, A. M. & Hasselmo, M. E. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Front. Integr Neurosci. 4, 2 (2010)
Jones, M. W. & Wilson, M. A. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial working memory task. PLoS Biol. 3, e402 (2005)
Sigurdsson, T., Stark, K. L., Karayiorgou, M., Gogos, J. A. & Gordon, J. A. Impaired hippocampal–prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464, 763–767 (2010)
Lee, I. & Kesner, R. P. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J. Neurosci. 23, 1517–1523 (2003)
Wang, G. W. & Cai, J. X. Disconnection of the hippocampal–prefrontal cortical circuits impairs spatial working memory performance in rats. Behav. Brain Res. 175, 329–336 (2006)
Hoover, W. B. & Vertes, R. P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 212, 149–179 (2007)
Jay, T. M. & Witter, M. P. Distribution of hippocampal CA1 and subicular afferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinine. J. Comp. Neurol. 313, 574–586 (1991)
Oh, S. W. A mesoscale connectome of the mouse brain. Nature 508, 207–214 (2014)
Burton, B. G., Hok, V., Save, E. & Poucet, B. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behav. Brain Res. 199, 222–234 (2009)
Jung, M. W., Qin, Y., McNaughton, B. A. & Barnes, C. L. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb. Cortex 8, 437–450 (1998)
Kjelstrup, K. B. et al. Finite scale of spatial representation in the hippocampus. Science 321, 140–143 (2008)
Royer, S., Sirota, A., Patel, J. & Buzsáki, G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J. Neurosci. 30, 1777–1787 (2010)
Chow, B. Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010)
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010)
Rigotti, M. et al. The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590 (2013)
Engel, A. K., Konig, P., Kreiter, A. K. & Singer, W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science 252, 1177–1179 (1991)
Carr, M. F., Karlsson, M. P. & Frank, L. M. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75, 700–713 (2012)
Yamamoto, J., Suh, J., Takeuchi, D. & Tonegawa, S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell 157, 845–857 (2014)
Vinck, M., van Wingerden, M., Womelsdorf, T., Fries, P. & Pennartz, C. The pairwise phase consistency: a bias-free measure of rhythmic neuronal synchronization. Neuroimage 51, 112–122 (2010)
Baeg, E. H. et al. Dynamics of population code for working memory in the prefrontal cortex. Neuron 40, 177–188 (2003)
Horst, N. K. & Laubach, M. Working with memory: evidence for a role for the medial prefrontal cortex in performance monitoring during spatial delayed alternation. J. Neurophysiol. 108, 3276–3288 (2012)
Funahashi, S., Bruce, C. & Goldman-Rakic, P. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J. Neurophysiol. 61, 331–349 (1989)
Goldman-Rakic, P. S. Cellular basis of working memory. Neuron 14, 477–485 (1995)
Seamans, J. K., Lapish, C. C. & Durstewitz, D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox. Res. 14, 249–262 (2008)
Rogers, D. C. et al. Photothrombic lesions of the frontal cortex impair the performance of the delayed non-matching to position task by rats. Behav. Brain Res. 49, 231–235 (1992)
Shaw, C. & Aggleton, J. The effects of fornix and medial prefrontal lesions on delayed non-matching-to-sample by rats. Behav. Brain Res. 54, 91–102 (1993)
Sloan, H. L., Good, M. & Dunnett, S. B. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav. Brain Res. 171, 116–126 (2006)
Izaki, Y., Takita, M. & Akema, T. Specific role of the posterior dorsal hippocampus-prefrontal cortex in short-term working memory. Eur. J. Neurosci. 27, 3029–3034 (2008)
Siapas, A. G., Lubenov, E. V. & Wilson, M. A. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151 (2005)
Lubenov, E. V. & Siapas, A. G. Hippocampal theta oscillations are travelling waves. Nature 459, 534–539 (2009)
Patel, J., Fujisawa, S., Berényi, A., Royer, S. & Buzsáki, G. Traveling theta waves along the entire septotemporal axis of the hippocampus. Neuron 75, 410–417 (2012)
O’Neill, P. K., Gordon, J. A. & Sigurdsson, T. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J. Neurosci. 33, 14211–14224 (2013)
Ruediger, S., Spirig, D., Donato, F. & Caroni, P. Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning. Nature Neurosci. 15, 1563–1571 (2012)
Fanselow, M. A. & Dong, H. W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19 (2010)
Komorowski, R. W. et al. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J. Neurosci. 33, 8079–8087 (2013)
Meyers, E. M., Freedman, D. J., Kreiman, G., Miller, E. K. & Poggio, T. Dynamic population coding of category information in inferior temporal and prefrontal cortex. J. Neurophysiol. 100, 1407–1419 (2008)
Abbott, L. F. & Dayan, P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 11, 91–101 (1999)
Barak, O. & Rigotti, M. A simple derivation of a bound on the perceptron margin using singular value decomposition. Neural Comput. 23, 1935–1943 (2011)
Anlauf, J. K. & Biehl, M. The AdaTron: an adaptive perceptron algorithm. Europhys. Lett. 10, 687–692 (1989)
Rosenblatt, F. Principles of Neurodynamics: Perceptrons and the Theory of Brain Mechanisms (Spartan, 1962)
Krauth, W. & Mezard, M. Learning algorithms with optimal stability in neural networks. J. Phys. A Math. Gen. 20, L745–L752 (1987)
Dietterich, T. G. & Ghulum, B. Error-correcting output codes: a general method for improving multiclass inductive learning programs. Proc. AAAI 91, 572–577 (1991)
Acknowledgements
The authors would like to thank M. Topiwala for technical assistance, M. Kheirbek for advice and assistance with the design of fibre optics, and M. Shapiro for advice with regards to designing the four-arm T-maze. This work was supported by grants from the National Institutes of Health (MH096274 and MH081968), the Hope for Depression Research Foundation, the International Mental Health Research Organization, the Gatsby Charitable Foundation and the Swartz Foundation.
Author information
Authors and Affiliations
Contributions
T.S., J. A. Gogos and J. A. Gordon designed the experiments. T.S. performed the experiments and analysed the data. M.R. and S.F. developed the linear classifier, adapted it for use with the T-maze data set, and provided guidance on its implementation. S.E.A. participated in the design of optogenetic experiments. T.S., S.F., J. A. Gogos and J. A. Gordon interpreted the results. T.S. and J. A. Gordon wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Individual mPFC units clustered from fibre-coupled stereotrodes.
a, Multiple individual units clustered from stereotrode recordings in the mPFC in the absence and presence of illumination. b, Mean waveforms of extracellular potentials from example units in a.
Extended Data Figure 2 mPFC cells encode goal location both categorically and globally.
a, A raster plot of spikes fired by an example single unit across trials, sorted by sample goal, temporally aligned to arrival at sample goal. b, Traces of firing rates averaged across trials by sample goal location, for the unit from a. This unit shows location selectivity, firing preferentially in the back left goal. Traces are mean ± s.e.m. c, Spatial map of firing rates for the same unit for the full recording session. Goal-selective units tended to fire more at the preferred goal than at the other goals, and more at all goals than in the rest of the environment. d, Percentage of units that were goal-selective as a function of time from sample goal, according to two-way repeated measures ANOVAs performed on binned spike rates. Units were identified as having selectivity for left/right (blue), back/front (red), and/or combined spatial dimensions (green). Dashed line represents chance (P = 0.05). Inset, percentage of units having each type and/or combination of selectivity at time zero (arrival at sample goal). Percentages are out of 792 recorded units.
Extended Data Figure 3 mPFC units represent choice goal location, not sample goal location, during choice runs.
a, Model accuracy at the time bin corresponding with arrival at the sample goal port during the four-goal task was highest for spike histograms with time bins of 500 ms and 1,000 ms. Five-hundred-millisecond time bins were used for spike analyses. b, Decoding sample goal location during subsequent choice run during the four-goal task. Using the linear decoder, previously visited location was not detectable above chance accuracy. Ten- and twenty-second delay trials were combined. c, Decoding choice goal during choice run, correct versus incorrect trials during the four-goal task. Location decoded for this analysis was chosen goal (that is, the mouse’s current location) rather than correct goal. Model accuracy reached 0.93 upon arrival at the goal on correct trials. On incorrect trials, model accuracy exceeded chance during goal approach but dropped to chance levels upon reaching the goal. Ten- and twenty-second delay trials were combined. d, Decoding choice accuracy (correct versus incorrect) during choice trials. Model accuracy peaked at 0.99 at 1.9 s after arrival at the goal. b–d, Histograms were aligned to departure from start box. Ten- and twenty-second delay trials were combined. Data show mean ± 95% confidence intervals for b and d; s.e.m. for c and e.
Extended Data Figure 4 vHPC–mPFC terminal inhibition does not alter mPFC spike rate.
a, Waveform features used to separate putative cell types. Spike duration was defined as the peak-to-trough time, while afterhyperpolarization (AHP) energy was taken as the area over the curve after the second zero-crossing. Spike duration yielded the clearest separation. b, Putative fast-spiking (FS) and non-FS cells, sorted by spike width, showed no effect of terminal illumination on spike rate (Arch– non-FS: sign rank z = −1.7, P = 0.095; Arch– FS: z = −1.6, P = 0.11; Arch+ non-FS: z = −2.7, P = 0.79; Arch+ FS: z = −0.49, P = 0.62).
Extended Data Figure 5 Effect of mPFC illumination on goal-selective firing in the mPFC.
a, Low-weighted units, as identified using the classifier, show no difference in firing between the goal with the highest weight relative to the other goals. In the sample goal these units fire at rates not different than their session mean rates. Traces indicate mean ± s.e.m. of normalized firing rate (bin FR − session FR). b, Terminal inhibition eliminates firing rate differences in preferred (Pref.) versus non-preferred (Other) goal during encoding across all units. On sample runs with no light, units from both Arch– (bottom left) and Arch+ animals (top left) had elevated firing rates in preferred goal relative to non-preferred goal (red asterisks mark time points with Bonferroni-corrected significance). In Sample Light runs, units from Arch– animals maintain elevated firing in the preferred goal (bottom right), while units from Arch+ animals show no significant firing rate difference (top right; N = 358 Arch– units, 325 Arch+ units, sign rank P < 0.0005).
Extended Data Figure 6 vHPC gamma modulates vHPC output.
a, vHPC units phase-lock maximally to the vHPC gamma rhythm at a lag of zero (P value from Rayleigh’s test < 0.05, dashed line indicates chance rate). b, Normalized PPC values, sorted by lag of maximal phase-locking, for significantly phase-locked vHPC units. Units with Bonferroni-corrected significance within the −40 to 40 ms lag window (Rayleigh test, P < 0.0029) were included. c, Mean normalized PPC value for the population shown in b. Shading is s.e.m. d, Histogram of units with maximum PPC value at each lag. Units maximally phase-locked at a lag of zero, with no net difference from zero across the population. e, vHPC units share a common preferred gamma phase. Pooled spikes from significantly phase-locked vHPC units were modulated by vHPC gamma phase at zero-lag (N = 26,303 spikes, Rayleigh’s z = 17.6, P = 2.2 × 10−8, PPC value = 0.002), with peak spiking in the descending phase of the gamma cycle. (Note that spikes and LFPs were both recorded from stereotrodes in the stratum pyramidale and that this gamma phase would probably differ from that recorded in SLM, as in Fig. 5).
Extended Data Figure 7 mPFC theta activity follows dHPC and leads vHPC during the task.
a, Example vHPC LFP (blue, right) and spectrogram (left) demonstrating robust theta (grey, 4–12 Hz) and gamma (red, 30–70 Hz) components during all runs towards goals. b, Pseudocolour plot of relative strength of mPFC unit phase-locking to vHPC theta at lags from −200 ms to 200 ms, for units with Bonferroni-corrected significance in at least one lag. Warmer colours indicate stronger phase-locking. c, Distribution of lags at peak phase-locking strength for significantly phase-locked mPFC units. Distribution centred at 0 (N = 189 units, z = 2.05, P = 0.98). d, Mean ± s.e.m. PPC value of mPFC units and vHPC theta, as a function of lag. e–g, Phase-locking of mPFC units to dHPC theta as a function of lag, as in b–d. Distribution of lags at peak phase-locking is significantly shifted towards a dHPC lead (N = 160 units, sign rank z = −4.4, P = 6 × 10−6). h, No difference in strength of phase-locking of mPFC units to vHPC (left) and dHPC (right) theta in light on versus light off trials. Mean and s.e.m. shown for each (N = 140 units, sign rank z = −1.3, P = 0.2; z = −1.4, P = 0.12). i–k, Phase-locking of vHPC units to mPFC theta as a function of lag, as in b–d. Distribution of lags at peak phase-locking is significantly shifted towards an mPFC lead (N = 51 units, z = −5.03, P = 2.4 × 10−7).
Supplementary information
Mouse engaged in the 4-goal T-maze task
This video shows a mouse engaged in the 4-goal T-maze task, with and without terminal inhibition by light aimed at mPFC. (MOV 10215 kb)
Rights and permissions
About this article
Cite this article
Spellman, T., Rigotti, M., Ahmari, S. et al. Hippocampal–prefrontal input supports spatial encoding in working memory. Nature 522, 309–314 (2015). https://doi.org/10.1038/nature14445
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14445
This article is cited by
-
Control of working memory by phase–amplitude coupling of human hippocampal neurons
Nature (2024)
-
Systematic characterization of a non-transgenic Aβ1–42 amyloidosis model: synaptic plasticity and memory deficits in female and male mice
Biology of Sex Differences (2023)
-
The Q/R editing site of AMPA receptor GluA2 subunit acts as an epigenetic switch regulating dendritic spines, neurodegeneration and cognitive deficits in Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Oligodendrocyte dynamics dictate cognitive performance outcomes of working memory training in mice
Nature Communications (2023)
-
Functional alterations of the prefrontal circuit underlying cognitive aging in mice
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.