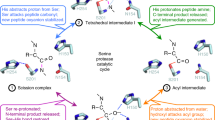
Abstract
Intramembrane proteases catalyse the signal-generating step of various cell signalling pathways, and continue to be implicated in diseases ranging from malaria infection to Parkinsonian neurodegeneration1,2,3. Despite playing such decisive roles, it remains unclear whether or how these membrane-immersed enzymes might be regulated directly. To address this limitation, here we focus on intramembrane proteases containing domains known to exert regulatory functions in other contexts, and characterize a rhomboid protease that harbours calcium-binding EF-hands. We find calcium potently stimulates proteolysis by endogenous rhomboid-4 in Drosophila cells, and, remarkably, when rhomboid-4 is purified and reconstituted in liposomes. Interestingly, deleting the amino-terminal EF-hands activates proteolysis prematurely, while residues in cytoplasmic loops connecting distal transmembrane segments mediate calcium stimulation. Rhomboid regulation is not orchestrated by either dimerization or substrate interactions. Instead, calcium increases catalytic rate by promoting substrate gating. Substrates with cleavage sites outside the membrane can be cleaved but lose the capacity to be regulated. These observations indicate substrate gating is not an essential step in catalysis, but instead evolved as a mechanism for regulating proteolysis inside the membrane. Moreover, these insights provide new approaches for studying rhomboid functions by investigating upstream inputs that trigger proteolysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brown, M. S., Ye, J., Rawson, R. B. & Goldstein, J. L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100, 391–398 (2000)
De Strooper, B. et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 (1999)
Bier, E., Jan, L. Y. & Jan, Y. N. rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes Dev. 4, 190–203 (1990)
Lichtenthaler, S. F., Haass, C. & Steiner, H. Regulated intramembrane proteolysis–lessons from amyloid precursor protein processing. J. Neurochem. 117, 779–796 (2011)
Chavez-Gutierrez, L. et al. The mechanism of γ-secretase dysfunction in familial Alzheimer disease. EMBO J. 31, 2261–2274 (2012)
Golde, T. E., Koo, E. H., Felsenstein, K. M., Osborne, B. A. & Miele, L. gamma-Secretase inhibitors and modulators. Biochim. Biophys. Acta 1828, 2898–2907 (2013)
Urban, S. Making the cut: central roles of intramembrane proteolysis in pathogenic microorganisms. Nature Rev. Microbiol. 7, 411–423 (2009)
Urban, S., Lee, J. R. & Freeman, M. A family of rhomboid intramembrane proteases activates all membrane-tether EGF ligands in Drosophila. EMBO J. 21, 4277–4286 (2002)
Baker, R. P., Wijetilaka, R. & Urban, S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Pathog. 2, 922–932 (2006)
Jin, S. M. et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942 (2010)
Morohashi, Y. & Tomita, T. Protein trafficking and maturation regulate intramembrane proteolysis. Biochim. Biophys. Acta 1828, 2855–2861 (2013)
Brooks, C. L. & Lemieux, M. J. Untangling structure-function relationships in the rhomboid family of intramembrane proteases. Biochim. Biophys. Acta 1828, 2862–2872 (2013)
Bhattacharya, S., Bunick, C. G. & Chazin, W. J. Target selectivity in EF-hand calcium binding proteins. Biochim. Biophys. Acta 1742, 69–79 (2004)
Baker, R. P. & Urban, S. Architectural and thermodynamic principles underlying intramembrane protease function. Nature Chem. Biol. 8, 759–768 (2012)
Fleig, L. et al. Ubiquitin-dependent intramembrane rhomboid protease promotes ERAD of membrane proteins. Mol. Cell 47, 558–569 (2012)
Dickey, S. W., Baker, R. P., Cho, S. & Urban, S. Proteolysis inside the membrane is a rate-governed reaction not driven by substrate affinity. Cell 155, 1270–1281 (2013)
Sampathkumar, P. et al. Oligomeric state study of prokaryotic rhomboid proteases. Biochim. Biophys. Acta 1818, 3090–3097 (2012)
Moldoveanu, T. et al. A Ca2+ switch aligns the active site of calpain. Cell 108, 649–660 (2002)
Moin, S. M. & Urban, S. Membrane immersion allows rhomboid proteases to achieve specificity by reading transmembrane segment dynamics. eLife 1, e00173 (2012)
Urban, S. & Baker, R. P. In vivo analysis reveals substrate-gating mutants of a rhomboid intramembrane protease display increased activity in living cells. Biol. Chem. 389, 1107–1115 (2008)
Xue, Y. & Ha, Y. Large lateral movement of transmembrane helix S5 is not required for substrate access to the active site of rhomboid intramembrane protease. J. Biol. Chem. 288, 16645–16654 (2013)
Strisovsky, K., Sharpe, H. J. & Freeman, M. Sequence-specific intramembrane proteolysis: identification of a recognition motif in rhomboid substrates. Mol. Cell 36, 1048–1059 (2009)
Bondar, A. N., del Val, C. & White, S. H. Rhomboid protease dynamics and lipid interactions. Structure 17, 395–405 (2009)
Fernandez-Chacon, R. et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 (2001)
Jaszai, J. & Brand, M. Cloning and expression of Ventrhoid, a novel vertebrate homologue of the Drosophila EGF pathway gene rhomboid. Mech. Dev. 113, 73–77 (2002)
Venturin, M. et al. Mental retardation and cardiovascular malformations in NF1 microdeleted patients point to candidate genes in 17q11.2. J. Med. Genet. 41, 35–41 (2004)
Koonin, E. V. et al. The rhomboids: a nearly ubiquitous family of intramembrane serine proteases that probably evolved by multiple ancient horizontal gene transfers. Genome Biol. 4, R19 (2003)
Sheiner, L., Dowse, T. J. & Soldati-Favre, D. Identification of trafficking determinants for polytopic rhomboid proteases in Toxoplasma gondii. Traffic 9, 665–677 (2008)
Feng, L. et al. Structure of a site-2 protease family intramembrane metalloprotease. Science 318, 1608–1612 (2007)
Li, X. et al. Structure of a presenilin family intramembrane aspartate protease. Nature 493, 56–61 (2013)
Urban, S. & Wolfe, M. S. Reconstitution of intramembrane proteolysis in vitro reveals that pure rhomboid is sufficient for catalysis and specificity. Proc. Natl Acad. Sci. USA 102, 1883–1888 (2005)
Baker, R. P., Young, K., Feng, L., Shi, Y. & Urban, S. Enzymatic analysis of a rhomboid intramembrane protease implicates transmembrane helix 5 as the lateral substrate gate. Proc. Natl Acad. Sci. USA 104, 8257–8262 (2007)
Acknowledgements
This work was supported by National Institutes of Health grant 2R01AI066025, the Howard Hughes Medical Institute, and the David and Lucile Packard Foundation. We are grateful to our colleague and friend A. Holland for use of his deconvolution microscope.
Author information
Authors and Affiliations
Contributions
S.U. and R.B. designed the research. S.U. performed all Drosophila cell biology experiments, while R.B. performed all biochemistry experiments. S.U. wrote the manuscript and R.B. made the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 The rhomboid-4 subfamily of rhomboid proteases.
a, ClustalW multiple sequence alignment of the conserved N-terminal EF-hand domains of 24 members of the rhomboid-4 subfamily (generated in Biology Workbench, http://workbench.sdsc.edu). Identical residues are shaded in green, highly conserved residues in yellow, and similar residues in cyan. The EF-hand calcium-binding loop consensus sequence is given below the alignment. b, Rooted tree of the 24 rhomboid-4 homologues. Genus and species names are colour-coded as follows: primates (blue), other mammals (green), birds (purple), fish (pink), non-vertebrate chordate (cyan), nematode (orange), and insects (red), with vernacular names given in parentheses, followed by National Center for Biotechnology Information (NCBI) accession numbers.
Extended Data Figure 2 Activity and thermostability analysis of DmRho4 mutants.
a, Comparison of calcium stimulation of DmRho4 versus its EF-hand domain deletion mutant (ΔEF), and a mutant lacking the entire cytosolic domain (ΔN). Upper diagram shows position of domains (demarked by residue numbers) and the corresponding deletion constructs. Transmembrane segments are shown as grey rectangles. GFP–Spitz substrate and cleavage products (green bands in the anti-GFP western) are denoted by black and white triangles, respectively. DmRho4 protein levels are shown as red bands (anti-HA western). b, Analysis of DmRho4 loop 2, 4, and 6 mutant protein levels from Fig. 2f (calcium stimulation conditions). c, DmRho4 loop 2, 4, and 6 mutants were assayed for cleavage of GFP–Spitz under basal (unstimulated) conditions for ∼24 h in the absence of calcium. Cleavage product (green bands, white arrowhead) was detected in media fractions for most of the mutants at levels comparable to the wild-type enzyme. Corresponding DmRho4 protein levels are shown as red bands (anti-HA western analyses). d, Wild-type DmRho4 and engineered variants were expressed and purified from bacteria, subjected to quantitative thermal stability analysis, and transition temperature midpoints (Tm) were derived (error bars, s.d. of four experimental replicates). The thermal stability of mutant DmRho4 proteases was indistinguishable from that of wild-type DmRho4.
Extended Data Figure 3 Calcium does not regulate DmRho4 through intermolecular interactions.
a, Anti-Flag co-immunoprecipitation analysis of HA–DmRho4 and APP–Spi7–Flag substrate from proteoliposomes in the presence or absence of 0.5 mM calcium. An inactive mutant of DmRho4 (S299A) was used to facilitate substrate complex isolation. The amount of HA-tagged DmRo4 co-immunoprecipitated with the Flag-tagged substrate was not affected by the presence of 0.5 mM calcium. L, load; B, bound. b, Anti-Flag co-immunoprecipitation of Flag–DmRho4 and HA–DmRho4 from proteoliposomes. HA-tagged DmRho4 failed to co-immunoprecipitate with Flag-tagged DmRho4 in both the absence and presence of 0.5 mM calcium. c, Mixing a catalytic mutant (H358A) and a calcium-binding mutant (E382A) cannot rescue calcium stimulation in trans (star indicates lane where a product would be expected with the mixed single mutants).
Extended Data Figure 4 Lateral substrate gating underlies direct regulation of intramembrane proteolysis.
a, Thermostability analysis of single and double cysteine mutants of DmRho4 (error bars, s.d. of four experimental replicates). b, Average relative proportions of cleavage at the external cleavage site (orange) compared with the internal cleavage site (blue) are shown for DmRho4 in the absence (no Ca) and presence (+ Ca) of 1 mM calcium (error bars, standard error of replicate experiments). The external site was favoured in the absence of calcium (approximately 80%) while internal cleavage was preferred in the presence of calcium (approximately 70%). c, DmRho4 loop 4 and loop 6 calcium-binding site mutants retained calcium-independent cleavage of a substrate harbouring only an external cleavage site. Full-length substrate (filled triangle) and cleavage product (open triangle) are indicated. d, Cleavage of a substrate with external and internal cleavage sites was compared for E. coli GlpG, P. stuartii AarA, and V. cholerae Rho1 in the absence (no Ca) or presence (+ Ca) of 0.5 mM calcium. The relative proportions of cleavage at the two sites varied between the bacterial rhomboid proteases, but in no case did calcium alter the cleavage site preference.
Rights and permissions
About this article
Cite this article
Baker, R., Urban, S. Cytosolic extensions directly regulate a rhomboid protease by modulating substrate gating. Nature 523, 101–105 (2015). https://doi.org/10.1038/nature14357
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14357
This article is cited by
-
Ten catalytic snapshots of rhomboid intramembrane proteolysis from gate opening to peptide release
Nature Structural & Molecular Biology (2019)
-
Relapsed acute lymphoblastic leukemia-specific mutations in NT5C2 cluster into hotspots driving intersubunit stimulation
Leukemia (2018)
-
Rhomboid intramembrane protease RHBDL4 triggers ER-export and non-canonical secretion of membrane-anchored TGFα
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.